Clinical guidelines are the cornerstone of current medical practice, providing high-quality, up-to-date advice for clinicians. Not only do they promote equity in clinical care through evidence-based medicine, but also they serve as important educational tools. In this article, we compare the most widely used clinical cardiology guidance by UK practitioners in the management of heart failure and cardiac device therapy – the National Institute of Clinical Excellence (NICE) and the European Society of Cardiology (ESC) guidelines.
Heart Failure
Heart failure is the inability of the heart muscle to contract or relax with full capacity and, therefore, unable to meet systemic circulatory demands.1 It affects approximately 64.3 million people worldwide and is one of the leading causes of morbidity and mortality.2 In the UK, heart failure affects 900,000 people and accounts for 5% of emergency hospital admissions, 1 million bed-days per year and up to £2 billion in annual costs.3,4
In developed nations, the most common aetiology for heart failure is ischaemic heart disease, followed by chronic hypertension, valve dysfunction and cardiomyopathy. Other causes are cardiac arrhythmias, infection and pericardial disease.5–7
Substantial advances have been made in the medical management of heart failure, a condition that has a notable impact on both the healthcare system and patients’ quality of life. In particular, sacubitril/valsartan and, more recently, sodium-glucose cotransporter 2 (SGLT2) inhibitors have been introduced. These have been shown to reduce mortality and hospitalisation and improve quality of life.8–10
Myocardial diseases are now understood better, leading to improved comprehension of heart failure aetiology. As such, diastolic dysfunction and impaired relaxation are increasingly being recognised as causes of symptomatic burden in the absence of reduced ejection fraction.11
The optimal management of heart failure involves reversing causation, medical therapy and cardiac implantable electronic device (CIED) implantation to improve cardiac function and reduce the risk of arrhythmic death.12–14
Cardiac Implantable Electronic Devices
Cardiac device therapy is an important and evolving domain in clinical cardiology, with new devices, technologies and techniques being developed. An estimated 1 million devices are implanted each year worldwide.15 In Europe alone, an estimated 637,000 devices are implanted, 60,000 of which were fitted in the UK.16,17
Cardiac implantable electronic devices include pacemakers, ICDs and CRT. Pacemakers are indicated in bradycardia in the presence of conduction system disease. This can range from sinus node dysfunction to complete atrioventricular nodal block. ICDs are indicated to overcome sustained malignant arrhythmias; ventricular tachycardia (VT) and VF are treated through either shock delivery or anti-tachycardia pacing in VT.
Finally, CRT devices are implanted to overcome dyssynchrony in patients with bundle branch block and severely impaired left ventricular systolic function. CRT has been shown to improve cardiac function and alleviate the symptomatic burden. These devices can have an additional defibrillation function when indicated.18
An increase in CIED implantation has been seen in view of an ageing population, better recognition of conduction system disease in young patients (inherited cardiac conditions, systemic inflammatory conditions and adult congenital heart disease) and widespread adoption of novel interventions such as transcutaneous aortic valve replacements (TAVR).18 This has been demonstrated by the steady increase in implantations between 2004 and 2014, with their number steadying with the stabilisation of life expectancy.17
Alternative CIEDs have emerged to overcome challenges faced by conventional therapies. Leadless pacemakers and subcutaneous ICDs offer an alternative approach where vascular access through the superior vena cava is limited or needs to be preserved for future access.
The first leadless pacemakers were sited in the right ventricle and limited to single-chamber, right-ventricular pacing; however, novel technology has shown atrioventricular synchronous pacing is now possible.19–21
In subcutaneous ICDs, the lead is placed in the extravascular space under the skin as opposed to within the right ventricle. Therefore, the device is unable to pace and is limited to patients with only an ICD indication.22–24
Finally, conduction system pacing uses the heart’s own conduction system to pace by careful placement of the lead in the His bundle or left bundle branch area. It is increasingly recognised as a more efficient method of pacing, with superior haemodynamic outcomes and symptomatic improvement in comparison to conventional pacing. As a result, this technique is being increasingly adopted.25–30
In the UK, cardiologists use both the National Institute of Clinical Excellence (NICE) and the European Society of Cardiology (ESC) guidelines to steer clinical management. In this review, we look at both guidelines’ approaches to heart failure and device therapy to discern the differences between the two and the impact these may have on clinical practice.
Discussion
NICE guidelines were established in 1999 to create consistency in medical practice and to overcome disparity between UK regions. NICE aims to optimise care in the NHS, a publicly funded healthcare system that spans primary to quaternary care.
The guidelines have the mammoth task of providing evidence-based advice across multiple specialities to health, public health and social care practitioners. NICE strives to achieve the most overall benefit to the greatest number of people by advising on high-quality, good-value care. In that regard, NICE prioritises cost-effectiveness in its guidelines.31
In contrast, the ESC guidelines focus on a single speciality: cardiovascular health. The society was founded in 1950 with the goal of publishing high-quality, evidence-based scientific knowledge to cardiovascular health professionals to reduce the burden of cardiovascular disease.
In addition to guidelines, ESC produces educational content and holds annual scientific congresses to better disseminate the most current knowledge.32
Medical Therapy
Guidance for heart failure requires a degree of careful consideration as it not only encompasses primary and secondary care settings but also guides management of both acute and chronic disease processes. ESC and NICE guidelines address these key elements individually.4,33,34
Both guidelines recommend regular use of ACE inhibitors, β-blockers and aldosterone antagonists as first-line medical therapy. However, a difference is seen regarding the inclusion of newer medical therapies.
Guidance on the use of sacubitril/valsartan is included in the NICE guidelines as the most recent update was in 2018, although SGLT2 inhibitors remain omitted.4 However, this has been addressed by NICE technology appraisal guidance and regional guidance on SGLT2 inhibitors, such as the Pan Mersey guidelines for Merseyside in north-west England. These specify indications, safety profile, costs, follow-up and monitoring.35,36 This does pose a risk to heterogeneity in delivery of SGLT2 inhibitors, as further guidance has to be sought in addition to reviewing the published NICE guidelines on heart failure management, which relies on the clinician’s awareness of the drug.
The NICE guidelines address cost and cost savings associated with implementation of the guidance in heart failure management and, in particular, the economic value of cardiac rehabilitation for these patients.4 Cost-effectiveness of sacubitril/valsartan and dapagliflozin were assessed using the incremental cost-effectiveness ratio (ICER) against quality-adjusted life years (QALY). Both drugs were within the accepted ICER per QALY of £20,000 and £10,000, respectively.37,35 Notably, cost is not covered by the European guidelines.33
Both ESC and NICE guidelines report the importance of a multidisciplinary approach to heart failure management, which includes a community team that comprises but is not limited to GPs, heart failure pharmacists and heart failure nurses.
Both touch on the management of concomitant renal disease in the presence of heart failure; this is a recognised problem in both acute and chronic heart failure management.
In addition, both guidelines press on the importance of palliative care in end-stage heart failure. Heart transplantation is a consideration in end-stage heart failure, which is not covered by NICE. ESC, however, does include this in its guidance, with additional information on bridging therapy and when this is indicated.4,33,34
NICE has established that a left ventricular ejection fraction greater than 50% indicates heart failure with preserved ejection fraction. However, there is little guidance on the identification and management of diastolic dysfunction and heart failure with preserved ejection fraction (HFpEF). Diagnosis and management of HFpEF is covered by the ESC guidelines.
Both guidelines describe the aetiology for heart failure, including ischaemic and valvular heart disease, hypertensive heart disease and cardiomyopathies. Management of comorbidities is also reported in both sets of guidance.4,33
Supplementary Material Table 1 provides a comparative summary of which aspects of management are included in the NICE and ESC guidelines in chronic and acute heart failure.
Cardiac Implantable Electronic Devices
The NICE guidelines for device management are reported as separate guidelines that include dual-chamber pacemakers, His bundle pacing, ICD, CRT, leadless pacemakers and subcutaneous ICDs.38–42 These guidelines were updated between 2014 and 2021.
However, the ESC guidelines on CIED were published in 2021 and cover all forms of cardiac devices in two documents, which cover: pacing and CRT; and therapies for ventricular arrhythmias (ICDs).18,43
Although the NICE guidelines do highlight the broad indications for dual-chamber pacing and touch upon where a single-chamber pacemaker can be considered, they remain reliant on the clinician’s judgement.
The institution does, however, detail the cost of each device early in the recommendations and, in turn, the overall cost implications for the NHS. This was approximately £43 million annually (at the time of publication). Similarly, the main indications for ICD and CRT with and without defibrillator capabilities are described.38,40
The costs are clearly defined for ICDs, CRT with pacing capabilities alone (CRT-p) and CRT with defibrillating capabilities (CRT-d). The difference between the cost of a CRT-p (£3,411) and a CRT-d (£12,293) is highlighted in this guidance.40 Much like with the novel heart failure medications, the cost-effectiveness of pacemakers, ICDs and CRT was assessed using ICER against QALYs. CRT-d or CRT-p and optimal medical therapy were determined as cost-effective in heart failure, falling within the committee’s accepted ICER per QALY gained of £30,000.38,40
Although the ESC guidelines do advise consideration of device cost during clinical decision-making, they do not delve into the specific costs themselves. There is little guidance on which devices are more expensive and to what degree, other than the higher costs of CRT being vaguely implied.18
The focus on scientific detail can be appreciated in the European guidelines. An example of the level of detail is seen in the number of itemised indications for bradycardia pacing with supporting evidence and the strength of the recommendation (Figure 1 and Supplementary Material Table 2).
There is also guidance on periprocedural considerations, which include but are not limited to antibiotic prophylaxis, operative techniques, complications including infection, MRI considerations and follow-up.
Finally, there is clear guidance on pacing and pacing modalities in differing QRS morphologies, which is limited in the NICE guidelines.18
A key difference between the two sets of guidance is the level of detail included. We use the number of itemised indications as an objective comparator of this difference (Figure 1 and Supplementary Material Table 2).
The NICE guidelines summarise bradycardia pacing in three items as: symptomatic sinus node dysfunction; high-degree atrioventricular block irrespective of symptoms; and high-degree atrioventricular block in the presence of AF.
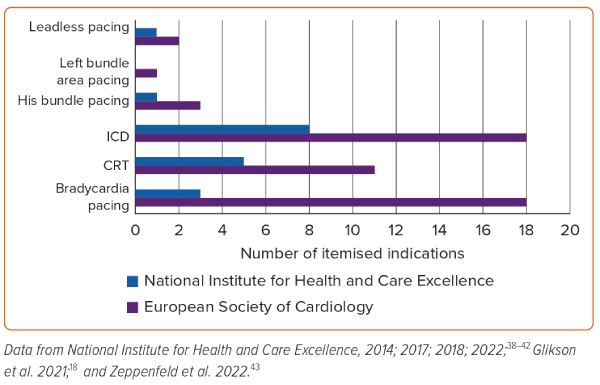
The ESC itemises these into 18 more descriptive components (Figure 1). Thus, these guidelines are more prescriptive and can be used by less experienced health professionals, not only to guide management but also as an educational tool.
The NICE guidance, in contrast, does require a degree of clinical expertise to exercise clinical judgement with the guidance used as support.
The indications and roles of novel technologies such as subcutaneous ICDs, leadless pacemakers and His bundle pacing are described by both guidelines.
However, leadless pacemakers and conduction system pacing remain under research governance in NICE guidance.18,39,41–43 Left bundle area pacing guidance is not included by NICE and the indication for His bundle pacing is limited to the treatment of heart failure only.39
In contrast, the ESC guidelines delve into more detailed specifics of the indications, benefits and roles of leadless pacemakers, conduction system pacing (divided into His bundle pacing and left bundle area pacing) and subcutaneous ICD insertion.18,43 This highlights the ESC’s aspiration to include the latest scientific information for cardiovascular healthcare professionals.
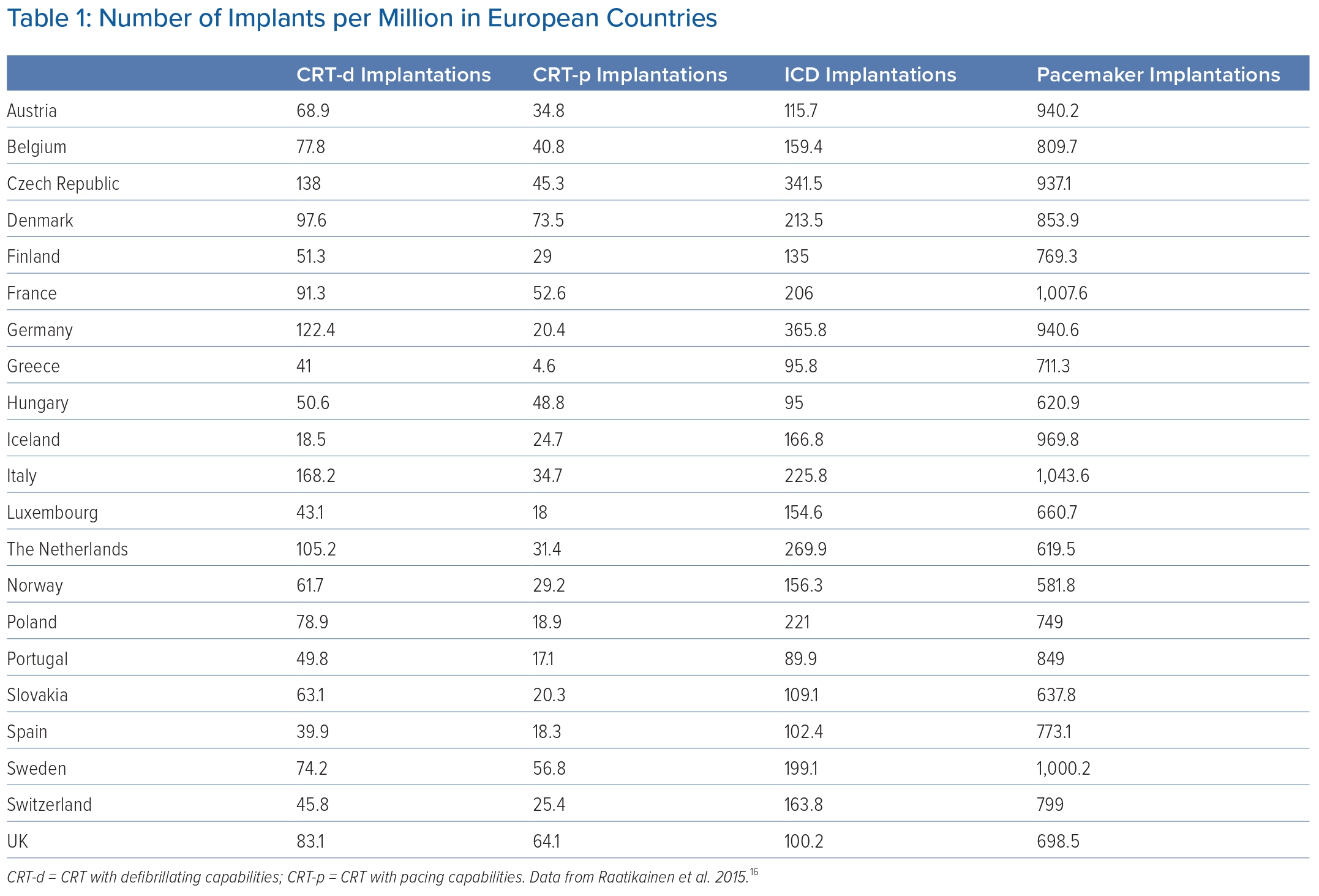
The impacts of the differences in the guidelines available to implanting cardiologists on clinical practice may be appreciated through the various implanting rates in different European countries.
In general, there is a higher rate of pacemaker implantation in equivalent-income European countries such as France, Germany and Italy compared to the UK (Table 1).16,44 Although the demographics of the paced populations are not available, the life expectancy in the listed countries appears to be similar at approximately 80 years. This loosely implies similar health burdens and environmental influences on the general population.45
One possibility for the disparity in the practice between the UK and Europe may be in the specificity of the advice within the two guidelines and the inclusion of cost in the NICE guidance. Implantation rates per million are lower in Great Britain at 698.5 per million than in Germany (940.6 per million), Italy (1,043.6 per million) and France (1,007.6 per million).16,18
Higher implant rates may have been expected in the UK because clinicians have a greater degree of freedom in considering implantation for more broadly defined indications.16,18,38 Alternatively, reporting costs transparently may influence decision-making, particularly when clinicians are weighing up the risks and benefits of an invasive prognostic treatment. As a result of high costs, there could be lower implantation rates. The cost of heart failure management and CIEDs are openly available to UK implanters in the NICE guidelines.38
The variations in CIED implantation rates across Europe have been reviewed previously, and socioeconomic factors are often touted as the main contributors to these disparities.46–48 For example, the UK and Germany have a similar GDP per capita (US$47,508 versus US$51,238, respectively), but the UK’s health spending was 39% less per head than in Germany between 2010 and 2019.49,50
Adoption of guidelines is the second leading factor, where cultural, economic and epidemiological obstacles impact implementation. Where there is little difference in the socioeconomic background and culture, a large gap remains in understanding the variations seen (for example, the difference in rates between UK and Germany). Here, it is presumed that rates are influenced by physician preference.48,49 Arguably, differences in how guidelines are presented can influence the development of personal preferences.
There is less variation in CRT implantation rates between countries and this may in part be due to better-defined indications in both the NICE and ESC guidelines that ultimately inform physician preference. This is despite CRT-d being the most expensive device (Table 1), which steers away from device cost being a leading influence in implantation disparity. This further suggests that clinical interpretation of available guidelines may impact on implantation decision-making.16,40,43
Indications for temporary pacing are given in the ESC guidance, which specifies the role of both transvenous and transcutaneous pacing. These are not included in NICE guidance, which encourages clinicians to exercise clinical judgement or consult ESC guidance.18
CIED in Heart Failure
Both NICE and ESC guidance are specific about the use of CIED for heart failure. Both use an ejection fraction of <35% as the marker of severely impaired left ventricular systolic function, at which point CRT is recommended in dyssynchronous contraction.
Where the ESC guidance specifies the indication for both left bundle branch block (LBBB) and non-left bundle branch block, NICE focuses on left bundle branch block alone. Furthermore, ESC guidance specifies the use of CRT over right ventricular pacing in patients with high-degree AV block and reduced ejection fraction and in those who undergo AV node ablation with heart failure. This guidance is not available with NICE.18,40
Indications for ICD implantation for primary and secondary prevention are included in both guidelines. These include with and without heart failure.18,40,43
Additional Guidance
Additional clinical guidance has been produced by American College of Cardiology (ACC), which is similar in detail to that provided by the ESC. Unlike the ESC guidance, these guidelines do discuss cost and cost-effectiveness assessed by ICER against QALYs. However, unlike NICE, these are not clearly itemised for each intervention.51
The Scottish Intercollegiate Guidelines Network also provides guidance for chronic heart failure management and this is accredited by NICE. These guidelines are extensive and include CIED management in heart failure. As they were updated in 2016, sacubitril/valsartan is included. Much like NICE, it highlights costs for each intervention and cost-effectiveness is measured using ICER against QALY.52
An advantage of having access to multiple guidelines is that each has its own strengths. Where NICE reviews the cost-effectiveness of medical management, ESC provides scientific detail.
Clinicians have the freedom to consult the most fitting guidance for the problem. As a result, they are better informed to make the most suitable clinical decision.
An example where multiple guidelines can be used is in the diagnosis of diastolic dysfunction; this is often referenced by ACC guidance, as it is perceived to have better algorithmic tools than NICE.
Conclusion
Cardiologists in the UK have access to both ESC and NICE guidelines to guide their practice for device intervention and heart failure management. The availability of both guidelines is important in guiding clinical practice; where ESC covers comprehensive scientific detail, NICE provides clear, itemised cost breakdown.
Practice varies between European countries, most notably in the number of pacemakers implanted per million people. Although speculative, this may in part result from socioeconomic factors (tariffs are considered in the UK) and in part be due to physician preferences influenced by available guidance. 
Click here to view Supplementary Material
Clinical Perspective
- Clinicians should understand the strengths and weaknesses of the two widely used clinical guidelines in heart failure and cardiac device management, issued by the National Institute of Health and Care Excellence and the European Society of Cardiology.
- Practice can be heterogenous due to differences between guidelines in the UK and Europe.
- Each guideline has strengths that can be used to improve clinical care.