Heart failure (HF) and AF are major causes of morbidity, hospitalisation and mortality. AF and HF with preserved ejection fraction (HFpEF) or reduced ejection fraction (HFrEF) frequently coexist due to complex pathophysiological interactions.1 More than half of HF patients exhibit AF during the course of their disease and more than one-third of AF patients exhibit incident HF.2 The association is even stronger with HFpEF, and AF-HFpEF patients exhibit a poorer prognosis compared to either condition alone.2–4 The management of AF in HFpEF is therefore a crucial issue for which the specificities of the AF-HFpEF population need to be taken into account.
Catheter ablation is a first-line treatment option for AF in selected patients. Randomised controlled trials (RCTs) have demonstrated its superiority to pharmacological treatment in terms of symptom and rhythm control, as well as prognostic outcomes including mortality, including in HFrEF.5,6 Nevertheless, there is a relative scarcity of data regarding the management of AF in HFpEF and the role of catheter ablation.
In the present non-systematic review, we discuss the role of catheter ablation in AF-HFpEF and the current evidence regarding its safety, efficacy and prognostic benefit.
Heart Failure with Preserved Ejection Fraction
HFpEF and HFrEF contribute equally to the burden of HF in the community, with HFpEF accounting for the majority of HF hospitalisations.7 HFpEF is a clinical syndrome that results from an inability of the heart to meet adequate cardiac output at normal filling pressures, in the absence of overt left ventricular (LV) systolic dysfunction at rest. The HFpEF population is phenotypically diverse because of substantial pathophysiological heterogeneity. Filling pressures are infrequently measured directly in clinical practice and the diagnosis of HFpEF is based on multimodal clinical workup showing signs and symptoms of HF, echocardiographic evidence of diastolic dysfunction and/or increased filling pressures and increased natriuretic peptide levels.8 In addition, isolated mimics of HFpEF, such as valvular heart disease, pericardial constriction and arrhythmias (including AF), need to be differentiated.
There is currently no established prognosis-modifying treatment in HFpEF, and evidence-based management is limited to diuretic drugs to alleviate signs and symptoms of congestion and screening of treatable comorbid conditions, therefore emphasising a pressing need to identify disease-modifying treatments.9
Current guidelines define HFpEF as LV ejection fraction (LVEF) ≥50%, HF with mid-range ejection fraction (HFmrEF) as LVEF 40–49% and HFrEF as LVEF <40%.8,9 Consistent with most AF-HFpEF literature, in the present review a preserved ejection fraction refers to an LVEF ≥50% unless otherwise specified; likewise, HFmrEF is grouped with HFrEF if not separately reported.
Epidemiology and Pathophysiology of AF-HFpEF
The prevalence of AF in the general adult population is approximately 3%, ranging from 0.1% among adults younger than 55 years to 9% among those older than 80 years.10–12 Similarly, HF exhibits an estimated 2% prevalence in the general adult population, rising to 8–10% in adults older than 75 years.1,13–15 Both AF and HF have an approximate lifetime risk of one in four to one in three.15–18 The incidence and prevalence of AF are rising in parallel to the growing burden of AF risk factors and population ageing. While the overall incidence of HF in high-income countries appears to have started to decline with an estimated peak in the mid-1990s, the prevalence seems stable or increasing, possibly related to improved HF survival up to recent years and population ageing.1,3,19–23 Importantly, the incidence of HFpEF has increased, in divergence from a decreasing incidence of HFrEF: an analysis on the Framingham Heart Study and Cardiovascular Health Study cohorts found that the age- and sex-standardised incidence of HFpEF rose from 4.7 to 6.8 per 1,000 person-years from the 1990–1999 decade to 2000–2009, while the standardised incidence of HFrEF declined from 6.6 to 6.2 per 1,000 person-years.23 This epidemiological shift may be related to a growing prevalence of HFpEF risk factors – including ageing – in parallel with improvements in the prevention and treatment of coronary artery disease, including a decline in ST-elevation MI.
AF and HFpEF or HFrEF are strongly associated, with AF occurring in more than half of HF subjects, and HF occurring in more than one-third of AF subjects.2 The bidirectional temporal relationship between AF and HF is apparent in the Framingham Heart Study. Among 382 individuals who were diagnosed with both AF and HF between 1948 and 1995, 38% developed AF first, 41% developed HF first, and 21% were diagnosed with both conditions on the same day.24 AF subjects exhibited an incidence of HF of 33 per 1,000 person-years, and HF subjects exhibited an incidence of AF of 54 per 1,000 person-years. The association is even stronger between AF and HFpEF. Data from major HF registries consistently show a higher prevalence of AF in HFpEF compared to HFrEF, ranging from 32 to 65% in HFpEF and 23 to 53% in HFrEF.25 In a more recent analysis from the Framingham Heart Study, among individuals with new-onset HF, the prevalence of pre-existing AF was higher in HFpEF than in HFrEF (32% versus 23%, respectively; p=0.002), and HFpEF subjects were more likely to exhibit AF at any time compared to HFrEF subjects (62% versus 55%; p=0.02).2 Interestingly, HFpEF and HFrEF were associated with similar risks of incident (future) AF. On the other hand, among individuals with new-onset AF, the rates of pre-existing HFpEF and HFrEF were similar. Among AF subjects who subsequently developed HF, 50% developed HFpEF, 40% developed HFrEF and 10% were unclassified. Interestingly, while AF individuals exhibited higher incidence rates of both HFpEF and HFrEF compared to non-AF individuals, multivariable adjustment for shared risk factors showed prevalent AF to be an independent predictor of incident HFpEF, but not HFrEF.
The association between AF and HF is related to complex and reciprocal pathophysiological mechanisms, the details of which are beyond the scope of this review. Briefly, AF and HF have a propensity to cause each other, in addition to sharing common risk factors that frequently contribute to the clinical picture; both conditions may also perpetuate or worsen each other over time in a mutually reinforcing fashion.
Shared risk factors include age, hypertension, diabetes, obesity, obstructive sleep apnoea (OSA), alcohol intake, chronic kidney disease (CKD), smoking and coronary artery disease (CAD).1,3,25 The high prevalence of comorbid conditions in AF-HFpEF can be appreciated in a large 2016–2017 real-word database in which, among 56,395 AF-HFpEF patients, 87.0% had hypertension, 41.7% had CAD, 33.8% had diabetes, 29.0% had stage ≥3 CKD, 28.6% had chronic obstructive pulmonary disease (COPD), 25.3% were obese and 15.8% had OSA.26 These risk factors cause alterations to atrial and ventricular function through inflammation, fibrosis, haemodynamic stress and ischaemia, resulting in structural, mechanical and electrophysiological remodelling.27,28 AF may cause or worsen HF through the loss of atrial systole and atrioventricular synchrony, rapid ventricular rates (including tachycardia-mediated cardiomyopathy per se), and irregular ventricular rhythm, leading in turn to reduced cardiac output, increased filling pressures and neurohormonal activation. Conversely, HF may cause AF through atrial mechanical stress and remodelling secondary to increased filling pressures, altered electrophysiological properties of the atrial tissue, abnormal calcium handling and neurohormonal and adrenergic activation.29
The epidemiological data from the Framingham Heart Study described above are compatible with a partially causal relationship between AF and HFpEF (prevalent AF independently predicts incident HFpEF), while shared risk factors appear to be a major driver for the association between prevalent AF and incident HFrEF (the association is lost after multivariable adjustment). Conversely, the similar risk of incident AF in HFpEF and HFrEF individuals could reflect that, when AF does not predate HF, the mechanisms that promote the development of AF in HF are partly shared between HFpEF and HFrEF.
While observational, these data provide a basic rationale for catheter ablation of AF as a disease-modifying intervention in HFpEF.
AF Leads to Adverse Outcomes in HFpEF
AF is an established predictor of mortality in HFrEF, as confirmed by several meta-analyses, and there is compelling evidence to support a similar effect in HFpEF.30–32
In the 1980–2012 Framingham Heart Study analysis, multivariable-adjusted analysis found that new-onset AF predicted higher all-cause mortality in both pre-existing HFrEF (HR 2.72; 95% CI [2.12–3.48]; p<0.0001) and HFpEF (HR 1.83; 95% CI [1.41–2.37]; p<0.0001), with a worse prognosis in pre-existing HFrEF compared to HFpEF (p for comparison=0.02).2 Similar results have been reported in several, but not all, HFpEF RCTs and registries and confirmed by meta-analysis.31 For example, in the Swedish Heart Failure Registry (SwedeHF), from 2000 to 2012, 9,595 patients had HFpEF (LVEF ≥50%), including 6,250 (65%) with AF at any point during follow-up and 3,345 (35%) without AF.33 Compared to sinus rhythm (SR)-HFpEF, and after multivariable adjustment, AF-HFpEF was associated with higher rates of all-cause mortality, HF hospitalisation, and stroke or transient ischaemic attack. Also notably, in the Americas component (n=1,765) of the TOPCAT trial, an RCT that randomised 3,445 HFpEF patients (LVEF ≥45%) older than 50 years to receive either spironolactone or placebo, both prevalent AF at enrolment (adjusted HR 1.34; 95% CI [1.09–1.65]) and incident AF post-randomisation (adjusted HR 2.53; 95% CI [1.80–3.55]) were associated with higher all-cause mortality over a mean follow-up of 2.9 years.4,34 Prevalent AF and incident AF were also associated with higher rates of the trial’s primary outcome (a composite of cardiovascular mortality, aborted cardiac arrest and hospitalisation for HF). The cause of excess death in AF-HFpEF was further assessed in another TOPCAT Americas post hoc analysis by Saksena et al., who compared 446 patients with AF on baseline ECG at inclusion with 1,319 patients with sinus rhythm (SR) at inclusion.35 Cardiovascular mortality at 5 years was much higher in AF-HFpEF patients compared to SR-HFpEF (30% versus 18%; p=0.014). Interestingly, pump failure death was more frequent in AF-HFpEF compared to SR-HFpEF (13% versus 5%; p=0.007), while the rate of sudden cardiac death was similar in both groups (10% versus 7%; p=not significant). These results may reflect the clinical impact of the adverse haemodynamic effects of AF, leading to worsening HF in the setting of HFpEF. Consistent with this hypothesis, Lam et al. reported that, compared to SR-HFpEF, AF-HFpEF patients exhibited lower peak oxygen consumption, higher N-terminal brain natriuretic peptide and higher left atrial (LA) volume index.36
Consistent with the findings from the TOPCAT trial, in which excess mortality associated with AF was greater for incident AF than prevalent AF, observational data on the temporal relationship between AF, HF and all-cause mortality have shown that new-onset AF carries a worse prognosis than prevalent AF, including in HFpEF patients.2,30
Considering this likely causal relationship between AF and HFpEF, the vicious circle of mutually reinforcing perpetuation and worsening that occurs when AF and HFpEF co-exist, and the adverse outcomes associated with AF-HFpEF, it can be reasonably hypothesised that efficacious – and preferably early – intervention to restore and maintain SR is likely to improve outcomes. The issue is particularly relevant given the very high prevalence of AF in HFpEF and the absence of established prognosis-modifying treatment in HFpEF.
Rhythm Control Versus Rate Control in AF-HFpEF
The current management of AF-HFpEF follows the principles of AF management in the general population and is based on the prevention of thromboembolism (anticoagulation), symptom management (rhythm control versus rate control) and risk factor management. The choice between rhythm control and rate control has been guided by patient symptoms and shared decision making, as RCTs comparing medical rhythm control to medical rate control have shown no prognostic benefit of rhythm control over rate control.37 However, contemporary RCTs that included catheter ablation as a rhythm control intervention showed improved cardiovascular outcomes in selected patients, including HFrEF5 and early AF (≤1 year), suggesting that rhythm control – and in particular by means of catheter ablation – may have prognostic benefit beyond symptom management.38,39 A meta-analysis that pooled data from 11 contemporary RCTs (3,598 patients) confirmed that catheter-based rhythm control resulted in lower all-cause mortality (OR 0.51; p=0.0003) and fewer hospital readmissions (OR 0.44; p=0.003) compared to medical rate control in HF (mostly HFrEF) patients.40 Consistent with historical data, medical rhythm control did not improve outcomes compared to medical rate control, and in fact was associated with higher hospital readmission rate (OR 1.25; p=0.01).
In contrast, there are no RCTs comparing rhythm to rate control in HFpEF. However, while conflicting data exist, observational evidence suggests prognostic benefit of rhythm control over rate control. In an analysis of the Get With The Guidelines-Heart Failure registry, Kelly et al. identified 15,682 patients aged ≥65 years who were discharged from hospital with a diagnosis of AF and HFpEF. At the time of discharge, 1,857 were treated with rhythm control and 13,825 received rate control.41 Patients receiving rhythm control were younger (median age 81 versus 83 years), but other baseline characteristics were similar. At 1 year, patients receiving rhythm control exhibited lower rates of all-cause mortality (30.8% versus 37.5%; p<0.01), all-cause readmissions (62.0% versus 64.6%; p=0.02), ischaemic stroke readmissions (1.56% versus 2.3%; p=0.02) and HF readmissions (26.3% versus 27.7%; p=0.05), compared to those receiving rate control. The association between rhythm control and survival was preserved after multivariable adjustment (adjusted HR 0.86; 95% CI [0.75–0.98]; p=0.02), while other outcomes were not independently associated with treatment strategy. In contrast, in a smaller observational cohort study that included 447 AF-HFpEF subjects, of whom 40 were treated with rhythm control, all-cause mortality over an average 4.1-year follow-up was not significantly reduced in the rhythm control group after propensity-score adjustment (adjusted HR 0.70; 95% CI [0.42–1.16]; p=0.16).42
In a retrospective observational multicentre study, Machino-Ohtsuka et al. compared 79 AF-HFpEF patients receiving rhythm control with 79 propensity-score-matched AF-HFpEF patients treated with rate control.43 Rhythm control was associated with a lower rate of a composite endpoint of cardiovascular death or HF hospitalisation (adjusted HR 0.30; 95% CI [0.18–0.98]; p=0.04) over a 24-month median follow-up. However, there were no significant differences in all-cause mortality and cardiovascular mortality between the two groups.
Catheter Ablation of AF in HFpEF
HF has been reported as a risk factor for arrhythmia recurrence after AF ablation.44,45 However, RCTs and subsequent meta-analyses of RCTs have demonstrated the superiority of catheter ablation compared to pharmacological rhythm and rate control in HFrEF for the reduction of all-cause mortality and hospital readmission over follow-ups of up to 60 months. 5,6,40,46,47 RCTs have also shown an improvement in LVEF following catheter ablation compared to medical management.5,46
In contrast, there are no dedicated RCTs assessing the safety, efficacy and prognostic benefit of AF ablation in HFpEF. However, substantial data are available from registries, observational studies, and post-hoc analyses of RCTs, from which meaningful insight can be derived for clinical practice and future research directions.
Safety and Efficacy
A growing body of observational data comparing HFpEF to HFrEF and/or to no HF indicates acceptable safety and efficacy profiles of catheter ablation of AF in HFpEF, that seem to mirror findings from HFrEF cohorts.48–57 For example, in a single-centre observational retrospective cohort study, Aldaas et al. analysed 547 patients who underwent de novo catheter ablation of AF, of whom 51 had HFpEF, 40 had HFrEF and 456 had no HF.50 HFpEF patients were more often female compared to HFrEF, exhibited a higher prevalence of hypertension and end-stage renal disease compared to no HF and a higher prevalence of OSA and COPD compared to both HFrEF and no HF. LA diameter, the prevalence of persistent AF and the prevalence of CAD were greater in HFpEF and HFrEF compared to no HF. Periprocedural complication rates did not differ significantly between groups and the rate of atrial arrhythmia recurrence at 5 years was similar. All-cause hospitalisation was more frequent in HF compared to no HF but did not differ significantly between HFpEF and HFrEF. All-cause mortality did not differ between groups. Importantly, these results were observed despite a higher prevalence of comorbid conditions and risk factors for post-ablation AF recurrence in HF participants.
Similar findings were reported by Yamauchi et al. in another single-centre observational retrospective study that included 502 consecutive patients who underwent non-paroxysmal AF ablation, of whom 293 had HFpEF, 84 had HFrEF and 125 had no HF.49 Compared to no HF and HFrEF, patients with HFpEF were older, more frequently female and had a higher prevalence of arterial hypertension. LA diameter and CHA2DS2-VASc score were higher in HFpEF and HFrEF compared to no HF. Of note, the proportion of patients with long-standing persistent AF was lower in HFpEF and HFrEF compared to no HF in this cohort. All-cause mortality at 1 year did not differ significantly between groups and no deaths occurred in HFpEF. There were no significant differences between groups in the rates of AF recurrence, repeat ablation, presence of SR at 1 year, and HF hospitalisation, with the exception of a higher rate of HF hospitalisation in HFrEF compared to no HF. Of note, the rate of 1-year AF-free survival in HFpEF was 83.6%, and 94.8% were in SR at 1 year.
A recent meta-analysis of six observational studies (three prospective, three retrospective) comparing 1-year outcomes of catheter ablation of AF in HFpEF versus HFrEF included a total of 1,505 patients (51% HFpEF, 49% HFrEF).58 There were no significant differences in the rates of periprocedural complications and hospitalisations. Mortality was lower in HFpEF compared to HFrEF (RR 0.41; 95% CI [0.18–0.94]; p=0.04). The risk of AF recurrence at 1 year did not differ between the two groups.
Prognostic Benefit of Catheter Ablation of AF in HFpEF
Despite the lack of dedicated RCTs, available evidence suggests that catheter ablation of AF in HFpEF individuals is not only safe and effective but also has prognostic benefit in terms of mortality and hospital readmission, as well as other markers of disease severity. Table 1 summarises the relevant studies.
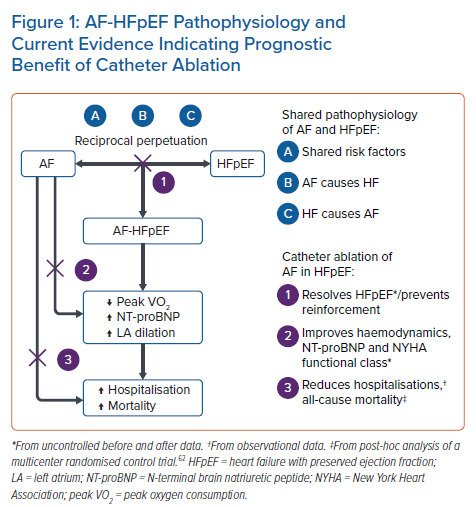
Firstly, uncontrolled before/after data provides evidence that SR restoration by catheter ablation may modify the course of the disease in AF-HFpEF, likely by altering the vicious circle of mutually reinforcing perpetuation of AF and HFpEF. Compared to baseline, catheter ablation was shown to result in lower natriuretic peptides levels, smaller LA diameter and area, lower rest and exercise pulmonary capillary wedge pressure, greater peak cardiac output, and improved New York Heart Association (NYHA) functional capacity at 6–12 months post-ablation.49,59,60 In addition, all-cause hospital admission rate has been reported to decrease by 28.5% (p<0.001) following catheter ablation, as compared to before ablation.57 In one study, assessment at 1 year post-ablation showed resolution of HFpEF (as per European Society of Cardiology diagnostic criteria) in 42.9% of participants after the first ablation procedure and in 51% after multiple procedures.9,59 Consistent with a causal effect, HFpEF resolution was strongly correlated with freedom from arrhythmia recurrence after ablation. In addition, atrial functional mitral regurgitation, a frequent occurrence in AF-HFpEF, has been found to improve significantly following AF ablation, likely as a result of reverse remodelling of the LA and of the mitral valve apparatus.61 Such haemodynamic improvements associated with favourable post-ablation reverse remodelling likely play a role in the clinical benefit observed following catheter ablation in AF-HFpEF.
Secondly, observational comparative data are consistent with a prognostic benefit of catheter ablation over medical therapy in terms of hospital readmission. In a single-centre retrospective comparative study, Fukui et al. analysed 85 consecutive patients who were diagnosed with HFpEF (LVEF ≥50%, clinical HF, and LV diastolic dysfunction) and AF.56 Fifty patients received medical therapy alone, and 35 patients with drug-refractory AF received catheter ablation. No major complications occurred. Freedom from arrhythmia recurrence did not differ significantly between the ablation group and the pharmacological rhythm control group (n=24). Nevertheless, over a mean follow-up of 792 days, catheter ablation was associated with a substantially lower rate of HF rehospitalisation (9% versus 48%, log-rank p=0.0039), as well as all-cause rehospitalisation (log-rank p=0.0284). Multivariable analysis showed catheter ablation to be the only independent predictor of freedom from HF rehospitalisation (OR 0.15; 95% CI [0.04–0.46]; p<0.001). Importantly, freedom from HF rehospitalisation was associated with SR maintenance (regardless of treatment arm) as compared to patients who exhibited arrythmia recurrence or who were in AF throughout the study (log-rank p=0.0185). Because catheter ablation was performed based on the presence of drug-refractory AF, likely selecting patients with a higher propensity to maintain AF, this finding supports a causal relationship between catheter ablation and freedom from HF rehospitalisation and renders confounding by indication unlikely.
Thirdly, randomised data have provided evidence for a reduction in mortality following catheter ablation compared to medical treatment. In a recently reported post-hoc analysis from the CABANA trial, a multicentre RCT which randomised 2,204 AF patients to catheter ablation or drug therapy, Packer et al. identified 778 patients with HF and functional capacity NYHA class II or greater, of whom 79% had HFpEF, 11.7% HFmrEF and 9.3% HFrEF.62 Subgroup intention-to-treat (ITT) analysis showed that, in HFpEF, catheter ablation resulted in markedly lower all-cause mortality at 4 years compared to drug therapy (3.3% versus 8.6%; HR 0.40; 95% CI [0.18–0.88]). It should be noted that this analysis was performed using imputation of missing baseline LVEF values. Subgroup analysis restricted to complete LVEF data (73% of the sample) showed no significant reduction in all-cause mortality in HFpEF (4.2% versus 8.3%; HR 0.51; 95% CI [0.23–1.12]). Nevertheless, full sample ITT analysis regardless of LVEF subgroup (but with an estimated 79% HFpEF) showed that, compared to drug therapy, catheter ablation resulted in a 44% relative reduction in first AF recurrence (HR 0.56; 95% CI [0.42–0.74]), as well as lower AF burden at all follow-up time points, sustained improvement in quality of life, lower rate of CABANA primary outcome (a composite of all-cause mortality, disabling stroke, serious bleeding or cardiac arrest; HR 0.64; 95% CI [0.41–0.99]) and lower all-cause mortality (6.1% versus 9.3%; HR 0.57; 95% CI [0.33–0.96]).
It should be noted that studies based on real-world data have reported conflicting findings. An analysis based on the Nationwide Readmissions Database from 2016–2017 failed to show prognostic benefit.26 Among 16,848 AF-HFpEF patients, of whom 1,053 underwent catheter ablation and 15,795 propensity-matched (1:15) controls did not, there was no significant difference in 1-year all-cause mortality, HF rehospitalisation and all-cause rehospitalisation between the two groups. However, AF rehospitalisation at 1 year occurred in substantially fewer patients in the ablation group compared to no ablation (4.9% versus 10.5%; HR 0.44; 95% CI [0.33–0.57]; p<0.001), which is compatible with clinically meaningful anti-AF efficacy.
It should also be noted that there are scarce data regarding the effect of AF ablation at different stages of HFpEF. Based on exercise right heart catheterisation, early HFpEF (i.e. exercise-induced only) has been defined as elevated LV filling pressures during exercise with normal LV filling pressures at rest (peak exercise pulmonary capillary wedge pressure ≥25 mmHg with resting pulmonary capillary wedge pressure <15 mmHg), while overt HFpEF – presumably representing more advanced disease – has been defined as elevated LV filling pressures at rest (resting pulmonary capillary wedge pressure ≥15 mmHg).63 When systematic HFpEF screening is performed among AF patients regardless of symptoms, the majority of AF-HFpEF patients have been found to exhibit early HFpEF (64–74%).60,64 In one study in which 74% of AF-HFpEF patients had early HFpEF, catheter ablation of AF resulted in a significant decrease in peak exercise pulmonary capillary wedge pressure, as well as lower natriuretic peptide levels and greater peak cardiac output.60 The available data therefore suggest prognostic benefit of AF ablation in early HFpEF. However, to our knowledge, there are no reports of ablation outcomes stratified by HFpEF stage, which is an issue that requires further study.
AF Catheter Ablation Strategies with Potential Benefit in HFpEF
Based on indirect evidence, AF ablation strategies that are likely to provide the most benefit in HFpEF should favour AF ablation early in the course of the disease and minimise the extent of LA lesions through individualised substrate modification.
The timing of catheter ablation in AF-HFpEF merits attention, as the prognostic benefit may be greater with early intervention. From a pathophysiological perspective, given the progressive nature of the AF-HFpEF vicious circle with progressively worsening LA/LV haemodynamics, AF burden and LA/LV remodelling, early disease-modifying intervention is likely to provide additional benefit. Moreover, as mentioned above, data from the randomised TOPCAT trial and observational data from large registries show that new-onset AF is associated with a worse prognosis than prevalent AF in HFpEF, emphasising this potential window of opportunity for early intervention.4,2,30 Finally, RCTs have shown additive prognostic benefit from early catheter ablation in a general AF population and in HFrEF.38,65 It is therefore likely that catheter ablation early after AF diagnosis in AF-HFpEF would provide greater prognostic benefit.
Likewise, the timing of catheter ablation with respect to the progression of HF may affect outcomes. The severity of HF, which may be assessed by the NYHA functional classification, is known to influence the efficacy, prognostic benefit, and risk/benefit ratio of several therapeutic interventions in HFrEF.9 NYHA functional class is also correlated with the prevalence of AF in HFrEF.66 Currently available data on catheter ablation in AF-HFpEF provide little insight into the subject and future studies are needed to assess possibly differential outcomes of AF ablation in different NYHA HFpEF subgroups. Furthermore, given the considerable pathophysiological heterogeneity of HFpEF, and heterogeneity in underlying electrophysiological AF substrate, management tailored not only to disease severity but also to specific aetiological subgroups is likely to improve outcomes.67
Regarding the benefit of minimising the extent of LA injury, there is consistent evidence showing that LA lesions from catheter ablation and the resulting scar formation may adversely affect LA reservoir, conduit, booster pump and neurohormonal functions.68 LA electromechanical synchrony may be impaired by iatrogenic conduction disturbances, and LA ejection fraction has been described to decrease proportionally to post-ablation scar volume.69,70 Importantly, LA compliance may be further reduced following catheter ablation, leading in extreme cases to increased filling pressures with pulmonary hypertension, an entity that has been referred to as stiff LA syndrome. In a retrospective study of 499 unselected patients who underwent AF ablation, 8.2% exhibited an increase in right ventricular systolic pressure (RVSP) >10 mmHg on echocardiogram with an RVSP >35 mmHg post-ablation.71 This outcome was associated with echocardiographic features of LV diastolic dysfunction. In contrast, in another study where 1,380 consecutive patients were prospectively assessed before and after catheter ablation for the occurrence of stiff LA syndrome, pulmonary hypertension with LA diastolic dysfunction confirmed by right heart catheterisation or direct LA pressure measurement was found in only 1.4% of patients and was not associated with LV diastolic dysfunction.72 It can be hypothesised that differing mechanisms may lead to increased pulmonary artery pressure following catheter ablation, namely the stiff LA syndrome proper (due to LA diastolic dysfunction) versus the development/unmasking of LV diastolic dysfunction. Both entities are of particular relevance to catheter ablation in AF-HFpEF. While the available data seem to indicate improvements in haemodynamic parameters, including a decrease in filling pressures and resolution of LV diastolic dysfunction following catheter ablation in AF-HFpEF, this issue merits continued assessment in future AF-HFpEF trials.59,60 In the meantime, careful attention should be given to minimise atrial injury when catheter ablation is performed.
Given the interindividual variability in non-pulmonary vein (PV) AF substrate, and suboptimal outcomes of PV isolation alone, a rational strategy to achieve minimal atrial injury while optimising rhythm outcomes should involve individualised non-PV substrate modification. Briefly, non-PV substrate modification strategies have used differing methods in attempts to identify and localise AF substrate, including low-voltage area ablation, complex fractionated atrial electrogram ablation, focal impulse and rotor modulation, dominant frequency mapping, AF ‘nest’ ablation and anatomically guided linear ablations.73–78 In addition to the identification of electroanatomical AF substrate per se, strategies to minimise the extent of atrial injury have used functional ablation endpoints, including AF termination and AF non-inducibility, to guide the extent of substrate modification.45 By analogy with other tachyarrhythmias for which persistent arrhythmia/inducibility at procedure end is associated with poor rhythm outcomes, AF ablation strategies guided by functional endpoints seek to demonstrate efficient elimination of the atrial substrate necessary to sustain AF. Crucially, such strategies may allow more selective substrate modification compared to systematic elimination of all identified ablation targets. Recent work from our group showed that inducibility of sustained AF by burst-pacing immediately after PV isolation (PVI) was associated with a higher rate of AF recurrence at 24 months.79 In a subsequent study based on sequential non-PV substrate modification (fractionated electrogram ablation) guided by stepwise AF inducibility testing, we showed that achievement of AF non-inducibility or termination during stepwise persistent AF ablation was associated with fewer AF recurrences at 24 months (HR 0.31; 95% CI [0.12–0.84]; p=0.021).80 Likewise, AF recurrence after catheter ablation was associated with progression in AF inducibility at repeat catheter ablation defined as persistently inducible AF at further steps of the redo procedure compared to the index procedure.81 These findings suggest that individualised and selective substrate modification guided by repeated AF inducibility testing may result in favourable rhythm outcomes while minimising the extent of LA injury. Consistent with this inference, our AF inducibility-guided stepwise catheter ablation approach resulted in 68% of paroxysmal AF patients and 29% of persistent AF patients to be treated by PVI alone, with an overall 82% and 76% rate of 24-month AF-free survival, respectively. In the absence of randomised trials, a rational approach to catheter ablation in AF-HFpEF should involve similarly individualised substrate modification, given the adverse effects of LA scarring in HFpEF. 79,80
Interestingly, novel non-thermal ablation technology such as pulsed field ablation may have less impact on LA compliance by triggering different tissue repair mechanisms. Nakatani et al. recently showed that, compared to thermal ablation (radiofrequency or cryoablation), pulsed field ablation was associated with similar decreases in LA reservoir and booster pump functions and larger late gadolinium enhancement volume at cardiac magnetic resonance in the acute phase (<3 hours after ablation), while recovery of LA mechanical function and disappearance of the majority of late gadolinium enhancement in the chronic stage (3 months after ablation) was observed.82 Of note, both alterations persisted in the chronic stage following thermal ablation. These findings suggest that tissue repair following pulsed field ablation involves less chronic fibrosis compared to thermal ablation, therefore resulting in better preservation of LA mechanical function.
Limitations
In the absence of dedicated RCTs, the prognostic benefit of AF ablation in HFpEF cannot be inferred with certainty. Data from observational studies, registries and post-hoc analyses should be interpreted with the usual caution. In particular, given the overlap between AF haemodynamic features and HFpEF diagnostic criteria, there is a risk of HFpEF overdiagnosis in AF patients, which may lead to biased data. RCTs comparing the outcomes of catheter ablation with medical management in HFpEF are therefore needed.36
Conclusion
Figure 1 summarises the pathophysiology of AF-HFpEF and the current evidence indicating a prognostic benefit of catheter ablation of AF in HFpEF.
The majority of HFpEF patients develop AF during the course of the disease and this occurrence is associated with adverse outcomes. Rhythm control may represent a disease-modifying treatment opportunity in AF-HFpEF, and observational data are indicative of prognostic benefit compared to rate control. While there are no RCTs comparing catheter ablation to medical management of AF in HFpEF, catheter ablation has been widely reported to have an acceptable safety and efficacy profile in HFpEF patients. In addition, data from observational studies, large registries and post-hoc analysis from RCTs suggest that catheter ablation reduces HFpEF severity, hospitalisation rates and all-cause mortality. Prospective RCTs are needed to confirm these results. Stratification by HFpEF aetiology, severity and timing of AF ablation is likely to provide insight towards the tailored management of the heterogeneous HFpEF population. Based on indirect evidence, it seems reasonable to currently favour early catheter ablation and to minimise the extent of atrial injury during ablation.
Clinical Perspective
- Up to 65% of heart failure with preserved ejection fraction patients develop AF during the course of the disease, and the occurrence of AF is predictive of worse outcomes, including pump failure death.
- Catheter ablation of AF in heart failure with preserved ejection fraction has an acceptable safety and efficacy profile that seems to mirror findings from HFrEF cohorts.
- Growing evidence suggests that catheter ablation of AF may lead to a decrease in heart failure with preserved ejection fraction severity, lower hospitalisation rate and lower all-cause mortality, but randomised trials are needed.
- Early catheter ablation may confer additive benefit, given the role of AF in disease progression.
- The extent of atrial injury during ablation should be minimised to avoid further reduction in left atrial compliance, which might worsen heart failure with preserved ejection fraction through increased filling pressures.