Percutaneous ablation for ventricular tachycardia (VT) was first attempted in 1983 and has rapidly evolved to become an important option for controlling recurrent VTs.1 Endocardial ablation remained the only percutaneous approach until epicardial access was introduced by Sosa et al. in 1996 and thereafter became progressively more available as an adjunctive strategy for the treatment of challenging arrhythmias.2 The improved arsenal of percutaneous technologies, and the advances in understanding VT substrates, paved the way for consideration of catheter ablation early on in the treatment of patients with VT.3 Despite the progress made in the field, some key questions regarding the role of percutaneous epicardial mapping and ablation in the treatment of VT remain: When should an epicardial approach be used in the treatment of VT? Should this depend on the disease substrate? Should it be performed during an endocardial mapping procedure, or after an endocardial ablation has failed? We will discuss criteria for selecting an appropriate approach to map and ablate VT, focusing on location and imaging of the arrhythmogenic substrate, specific situations where an epicardial approach may be beneficial and situtations where it is not indicated.
Identification of the Arrhythmogenic Substrate and Ventricular Tachycardia Ablation – Patients With Structural Heart Disease
Understanding the underlying arrhythmogenic substrate that leads to VT is essential for a successful ablation procedure. As a result of the 3D structure of the myocardium, critical components of the VT reentry circuit may be confined to locations deep in the subendocardium, the midmyocardium or the epicardium, and may be beyond the reach of current endocardial ablation techniques. On the other hand, a single circuit may also encompass the endocardium and extend into the epicardium, with critical components that can be reached from either the left ventricular endocardium or the epicardium. Although VTs originate from scar tissue in the majority of patients with structural heart disease,4 they may occasionally have a focal source and can arise from the outflow tract region, similar to patients with idiopathic ventricular arrhythmias.
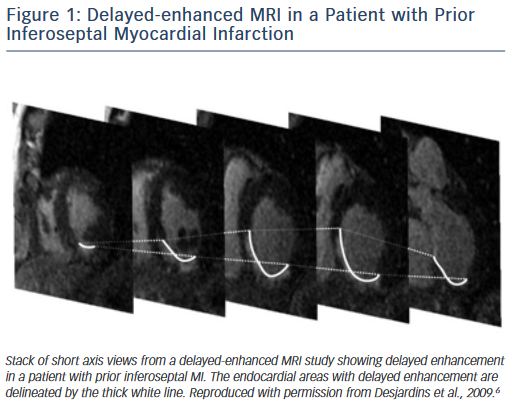
Delayed enhanced cardiac MRI is used as the gold standard for imaging of scar tissue. The location of scar tissue on preprocedural MRI determines the odds of reaching the circuit from the endocardium or the epicardial space in a given patient. The location of the scar predicts, to some extent, the location of the critical VT isthmus.5,6 In patients with ischaemic cardiomyopathy, the scar is located in the subendocardium and extends to the epicardium depending on the patient’s specific coronary artery distribution (Figure 1). Hence, in the majority of postinfarction patients, the arrhythmogenic tissue can be accessed from the endocardium. In a series of 98 patients with prior MI, only two patients required an epicardial ablation procedure for VT.7 Similar data have been described by Sarkozy et al. who reported a prevalence of only about 6 % of patients with prior infarctions in whom epicardial circuits were confirmed.8 Therefore, in these cases, an endocardial approach should be considered as first line.
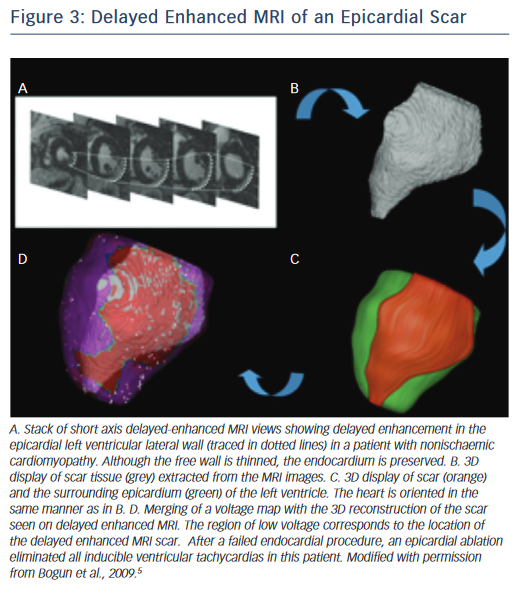
On the other hand, scars in patients with nonischaemic cardiomyopathy are most often located intramurally. An epicardial origin is the next most frequent scar location, followed by endocardial scarring.9 Often the scar has a basal periannular distribution.10 The scar distribution is particularly important with respect to procedural outcome.5 The lowest success rates were reported in patients in whom the majority of the scar had an intramural distribution (Figure 2).5 The value of a preprocedural MRI with respect to a planned epicardial procedure is highest in patients with nonischaemic cardiomyopathy, especially if the scar is located epicardially (Figure 3). An epicardial approach is also beneficial if the scar is located intramurally within the left ventricular free wall. An intramural septal location (Figure 2) cannot be reached from the epicardial space in most patients, and therefore this approach should not be used in such a scenario.
The disease process in arrhythmogenic right ventricular cardiomyopathy (ARVC) starts in the epicardium, and therefore the epicardium is usually involved in these patients. Furthermore, the epicardial scar has been found to be larger than the endocardial scar, and therefore an epicardial procedure is often required for patients with ARVC. If an endocardial ablation procedure fails to identify critical VT sites, an epicardial approach should be considered. Transmural activation of the epicardium from the endocardium may be delayed due to scar tissue that insulates the arrhythmogenic tissue in the epicardium.11 Often the paravalvular area of the tricuspid annulus harbours critical sites of VT circuits. Thick endocardial scarring has been described in the periannular area, and may contribute to the failure of endocardial ablation in targeting VT reentrant circuits.12 Involvement of the left ventricle is not uncommon, and is frequently subepicardial or intramural.13 Often, to render patients with ARVC non-inducible, both epicardial and endocardial ablation procedures are necessary.
In patients with cardiac sarcoidosis, the arrhythmogenic substrate originates intramurally, and reaches the endocardium or epicardium by extension of the disease process. In a series of patients with cardiac sarcoidosis in whom mapping and ablation of VT was performed, most VTs originated from a periannular area surrounding the tricuspid annulus and did not require epicardial access.14 However, if imaging shows predominant epicardial scarring, an epicardial approach should be considered.5
For other types of structural heart disease including hypertrophic cardiomyopathy15 or Chagas disease, an epicardial ablation is often necessary and should be considered early in the ablation procedure.16,17
Location of the Arrhythmogenic Substrate and Ventricular Tachycardia Ablation – Patients Without Structural Heart Disease
The majority of idiopathic ventricular arrhythmias originate from the outflow tracts18 with the right ventricular outflow tract being the most common origin.19,20 Idiopathic ventricular arrhythmias can also originate in the left ventricular outflow tract, the aortic sinuses of Vasalva,21 the epicardium,3 the papillary muscles22 or any other location within the heart including the intramural myocardium.23 An endocardial approach is most successful for the majority of these arrhythmias. For epicardial idiopathic arrhythmias, a combined approach from the coronary venous system and adjacent anatomic sites has been most effective, with a success rate around 70 %.24 In some reports, a subxiphoid epicardial approach has been used to eliminate those arrhythmias.24–26 However, several groups have more recently demonstrated that this approach has a lower success rate due to epicardial fat and the proximity of coronary arteries, and given the higher potential for periprocedural complications,26–28 this approach should be reserved for selected cases only.
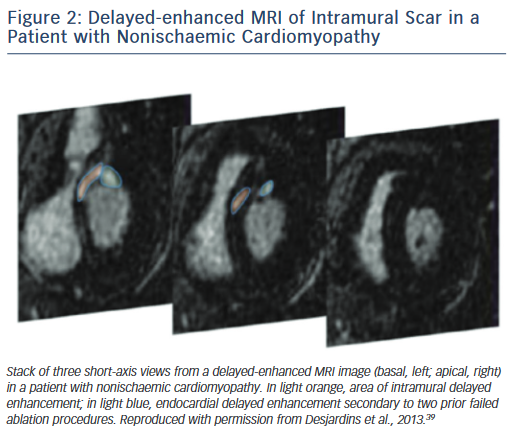
In summary, the type of structural heart disease and the resulting location of arrhythmogenic substrate indicate in most patients whether an epicardial procedure is necessary at the outset.
Preprocedural Considerations
A cardiac MRI with delayed enhancement should be obtained prior to an ablation procedure if at all possible. Post-processing of the images can be used to integrate the endocardial, epicardial and scar contours in 3D reconstruction images. There is a high degree of correlation between electroanatomical voltage mapping and the scar as characterised by MRI (Figure 3).6 Intraprocedural registration of the scar into the electroanatomic map helps to focus on an area of interest and facilitates the ablation procedure.29 The location of scar tissue has been correlated with the location of the arrhythmogenic substrate.5 A recent study demonstrated that preprocedural MRIs in patients with failed VT ablations also helped to localise the arrhythmogenic tissue and thereby indicated the likelihood of whether an epicardial ablation was necessary.30 In this study, patients with cardiac implanted electronic devices (CIEDs) were included. With appropriate precautions and device programming no adverse events were noted in the patients with CIEDs. Artefact production by the ICD generator is a limitation of MRI, as it can obscure parts of the heart, and scarring cannot be excluded with certainty if this is the case. Reduction of artefact, however, has been described using a novel wideband late gadolinium enhancement imaging protocol.31 Although MRIs have been described to be safe in patients with CIEDs32–34 it needs to be pointed out that such an approach is not yet standard of care in most institutions. Most patients with structural heart disease and VT will already have a CIED in place and therefore imaging of these patients might be more difficult depending on institutional practices and protocols. To safely perform imaging in these patients, a protocol detailing specific programming steps of the CIED prior and after the MRI and monitoring of the patient throughout the MRI in addition to specific exclusion criteria are necessary.
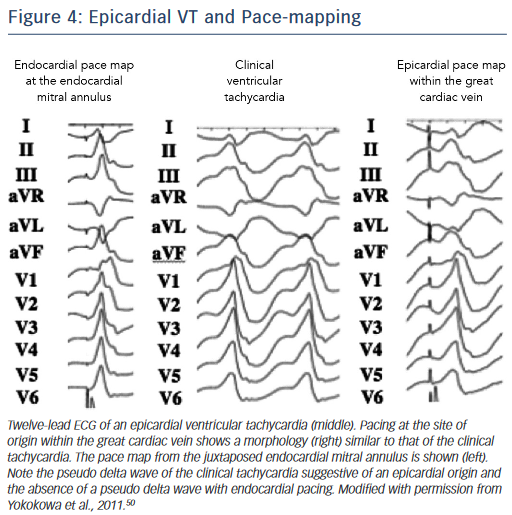
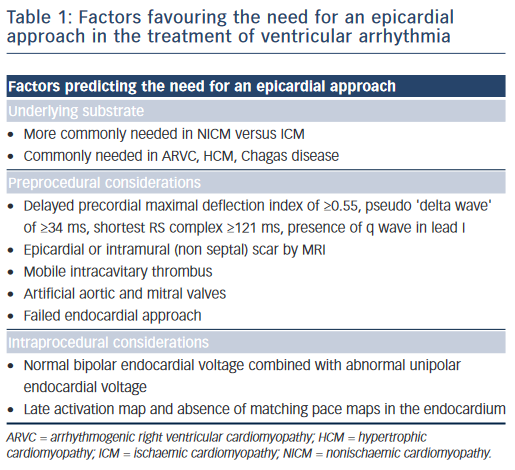
A preprocedural echo should rule out an intracavitary thrombus prior to a planned endocardial ablation procedure. ECG criteria have also been used to distinguish endocardial from epicardial origins.35 A delayed precordial maximal deflection index ≥0.55,25 a pseudo ‘delta wave’ at QRS onset ≥34 ms,36 a minimal RS interval ≥121 ms and the presence of a Q wave in lead I35,37 suggest an epicardial VT origin (Figure 4). Those ECG criteria are readily available and should be considered when predicting the VT exit site. However, their accuracy is limited, especially in patients with structural heart disease, and can be improved when using a computerised algorithm focusing on the ECG slope of the initial part of the QRS complex.38 It is essential to note that the QRS morphology solely reflects the VT exit site and cannot exclude the presence of endocardial components of the circuit, which may be eliminated using an endocardial approach.
Intraprocedural Considerations
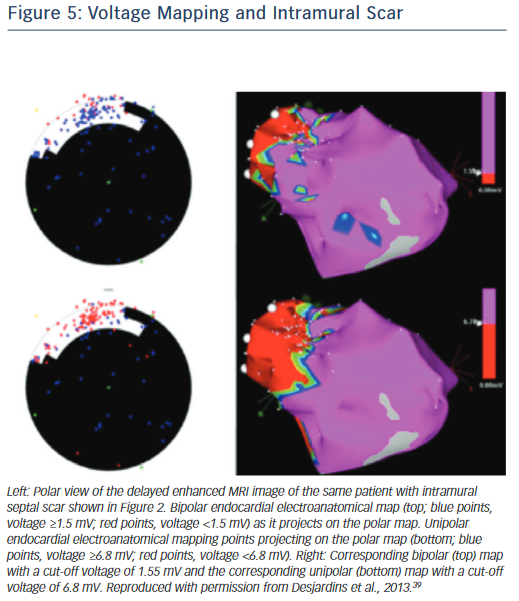
If endocardial mapping is performed first, in the absence of preprocedural imaging, unipolar mapping can be beneficial to indicate the presence of intramural or epicardial scar. While bipolar mapping is best in assessing local electrograms originating from tissue adjacent to the recording electrodes, unipolar mapping enables recording of deeper tissue layers. Unipolar signals are recorded between the distal tip of the ablation catheter and the Wilson central terminal during baseline rhythm. Unipolar endocardial mapping has been used for identification of nonendocardial scar that is located intramurally or epicardially. Different voltage cut-off values have been reported for identification of intramural scars in patients with nonischaemic cardiomyopathy (Figure 5),39 as well as epicardial scars in patients with ARVC40 and nonischaemic cardiomyopathy.41 In the presence of a normal endocardial bipolar voltage map combined with an abnormal unipolar voltage map an intramural or epicardial substrate needs to be considered and an epicardial approach may be warranted. Identification of intramural septal scarring is of key importance, as an epicardial procedure is unlikely to reach the intramural septal substrate from the epicardial space. Transseptal conduction times have been found to be prolonged, and may indicate intramural septal scarring.42 In patients with prior MIs, but also in patients with other structural heart diseases and transmural scar, it is possible that parts of the reentrant circuit can be reached from sites other than the endocardium. A recent study pointed out that in approximately onethird of patients with prior infarctions, critical VT sites identified in the endocardium were non-exit sites (i.e. the VT exit in these patients was not confined to the endocardium and was located elsewhere, in the epicardium or intramurally), whereas the remaining critical sites had the exit within the endocardial myocardium.43 This further supports our contention that in patients with prior infarctions, an endocardial approach should be the preferred initial method.
In patients with idiopathic outflow tract arrhythmias, late activation times in the absence of matching endocardial pace maps at the sites of earliest activation suggests a deeper or epicardial focus. Mapping within the aortic valve cusps and the coronary venous system is most helpful to identify an epicardial source. The subxiphoid epicardial approach, as mentioned above, is rarely successful in this scenario and should be limited to select cases given the higher risk of complications.26
Other Considerations
A failed endocardial ablation procedure can suggest that the critical area might not be reachable from the endocardium. In these patients, preprocedural imaging is particularly helpful, especially if an intramural or epicardial substrate can be demonstrated.
Elimination of all inducible VTs should be the optimal goal for an ablation procedure if this can be safely achieved, as this approach has the best long-term outcome. If VTs after an endocardial approach remain inducible, an epicardial approach should be considered.44 This approach has been beneficial in patients with prior MI, and also in patients with ARVC.
Patients with a mobile intracavitary thrombus, and those with left ventricular scarring who have implanted artificial aortic and mitral valves are not candidates for extensive endocardial mapping procedures. Alternative approaches (i.e. epicardial ablation, transcoronary ethanol ablation45,46) can be considered in such settings
Lesion depth is a major limitation for VTs originating from intramural sites. An epicardial procedure should be considered in the presence of intramural left ventricular free wall scarring. Bipolar ablation procedures from both aspects of the scar,47 ablation with an extendable needle catheter48,49 or simultaneous unipolar ablation49 have been described in this context. For these situations, epicardial access is helpful.
Conclusion
In our opinion, the choice between an endocardial versus an epicardial approach to target VT should depend on the patient’s underlying disease substrate, and the location of the arrhythmogenic substrate within the myocardial wall, which can be best assessed with a cardiac MRI. Certain patient-specific characteristics including ECG criteria, previously failed ablations, the presence of intracardiac thrombi or intraprocedural mapping data may further impact on the decision to proceed with an epicardial mapping and ablation approach. In patients with idiopathic outflow tract arrhythmias, a transcutaneous subxiphoid approach is rarely needed, even if the arrhythmia has an epicardial origin.
Clinical Perspective
- Critical components of a ventricular tachycardia (VT) reentry circuit may be confined to locations deep in the subendocardium, the midmyocardium or the epciardium, and may be beyond the reach of current endocardial ablation techniques.
- ECG criteria suggesting an epicardial VT exit site include a delayed precordial maximal deflection index of ≥0.55, a pseudo ‘delta wave’ QRS of ≥34 ms, the shortest RS complex of ≥121 ms and the presence of q wave in lead I. While these criteria may be helpful in patients with idiopathic arrhythmias, their value in patients with structural heart disease has been debated.
- The type of structural heart disease and the resulting location of the arrhythmogenic substrate indicate, in most patients, whether an epicardial procedure will be necessary.
- The value of a preprocedural MRI with respect to a planned epicardial access is highest when an epicardial or intramural scar is identified within the left ventricular free wall, especially in patients with nonischaemic cardiomyopathy.
- For idiopathic outflow tract ventricular arrhythmias, a coronary venous approach combined with an endocardial method is most beneficial. The subxiphoid epicardial access is rarely beneficial in these patients.