AF is the most common sustained arrhythmia encountered in clinical practice. In 2010, the global AF population was 33.5 million. It is estimated that the number of AF cases will increase by approximately 5 million people per year, with annual cases likely to double by 2030.1 AF increases the risk of stroke fivefold and increases the risk of morbidity and mortality.2 Among the largest contributing factors for the rapid increase in the incidence of AF are aging and obesity.3 Risk factors independently associated with AF that are linked to obesity include hypertension, diabetes, obstructive sleep apnoea (OSA), alcohol consumption and poor cardiorespiratory fitness.4 The aim of this article is to provide practical advice for clinicians seeking to manage patients with AF and OSA.
Obese individuals are more susceptible to repeated closure of the upper airway, known as OSA, during sleep. Additional risk factors include male sex and increasing age. Typical symptoms include snoring, fatigue, disrupted sleep and excessive daytime sleepiness. Continued nightly intermittent airway obstruction leads to large swings in negative intrathoracic airway pressure, intermittent hypoxia, repeated arousals from sleep and neurohumoral activation, all of which contribute to adverse cardiovascular events.5–7 Obstructive respiratory events result in an increased occurrence of spontaneous premature atrial contractions, which are potent triggers for AF episodes. In addition, airway obstruction promotes left atrial dilatation, slows atrial conduction and shortens the atrial effective refractory period, all of which make the atrium more susceptible to AF.7,8 OSA has also been shown to increase systemic inflammation, which may further predispose to the initiation of AF.7
Relationship Between Obstructive Sleep Apnoea and AF
There is a clear relationship between OSA and AF. Patients with OSA are two- to fourfold more likely to develop AF.9 The prevalence of nocturnal AF in patients with OSA has been estimated to be between 3% and 5%, which is higher than the prevalence in the general population. OSA is considered an independent risk factor for AF (HR 2.18; 95% CI [1.34–3.54]).4 Whether the risk of developing AF is proportional to the severity of OSA remains unclear. Nonetheless, the most recent European Society of Cardiology (ESC) guidelines advocate opportunistic screening for AF in patients with OSA (class of recommendation: IIa; level of evidence: C).10 The prevalence of OSA in patients with AF is 21–74%, which provides support for this recommendation.11
Current guidelines advocate addressing risk factors such as obesity and OSA in all patients with AF. In support of this, the LEGACY study demonstrated that weight loss alone can reduce the burden of AF and promote maintenance of sinus rhythm.12 In fact, weight loss ≥10% resulted in a sixfold greater probability of arrhythmia-free survival. In addition, the importance of weight maintenance was further emphasised, because patients whose weight fluctuated >5% were twice as likely to have AF recurrence.12
With respect to OSA, before it can be treated it has to be diagnosed. Prior guidelines have advocated interrogation for clinical signs of OSA in all AF patients.4 Unfortunately, the optimal method to determine which patients require sleep testing remains undefined.
Screening Questionnaires
Numerous screening questionnaires have been developed to screen for patients at risk for OSA and determine who should be referred for polysomnography (PSG), also known as a sleep study.
Commonly used questionnaires include the Epworth Sleepiness Scale (ESS), the Berlin questionnaire and the STOP-BANG questionnaire (Table 1).13-15 Unfortunately, the sensitivity and specificity of these questionnaires in AF patients remain poorly defined. Ideally, all patients with AF would undergo PSG; however, in the US, this is usually not possible due to constraints imposed by insurance coverage regulations. In our own experience of consecutive patients undergoing PSG, 85% had OSA and approximately 50% had moderate to severe OSA.
Epworth Sleepiness Scale
The ESS is a self-reported scale that evaluates the level of daytime sleepiness. Patients are asked to rate their ‘chance of dozing’ on a scale of 0–3 (0, would never dose; 1, slight chance of dozing; 2, moderate chance of dozing; 3, high chance of dozing) in eight different situations that are commonly encountered in daily life. The sum of the eight ratings gives the ESS score, which ranges from 0 to 24. ESS scores ≥10 suggest significant daytime sleepiness and would warrant a sleep study.13
Berlin Questionnaire
The Berlin questionnaire incorporates history of snoring, degree of tiredness, witnessed apnoeas, history of hypertension and obesity. Each question is within one of three categories, and each category has its own point system. The first category asks questions regarding snoring and witnessed apnoeas; the second category examines the level of tiredness; and the third category asks whether a patient has high blood pressure and incorporates their BMI. For this questionnaire, a BMI ≥30 kg/m2 is considered high risk. A patient is categorised as being low risk for OSA if they have one or no categories with a positive score and high risk if two or more categories are positive.14
STOP-BANG Questionnaire
The STOP-BANG questionnaire consists of eight questions that are answered with either a ‘yes’ or a ‘no.’ The questions evaluate the presence of loud snoring, tiredness, observed apnoeas, BMI ≥35 kg/m2, age >50 years, large neck circumference (≥43 cm in men and ≥41 cm in women) and male sex. Each question answered ‘yes’ is given 1 point; thus, the aggregate score ranges from 0 to 8. A person with a score of 0–2 is considered to be at low risk of OSA, whereas those with a score of 3–4 are considered to be at moderate risk and those with scores ≥5 are considered to be at high risk.15
Diagnosing Obstructive Sleep Apnoea
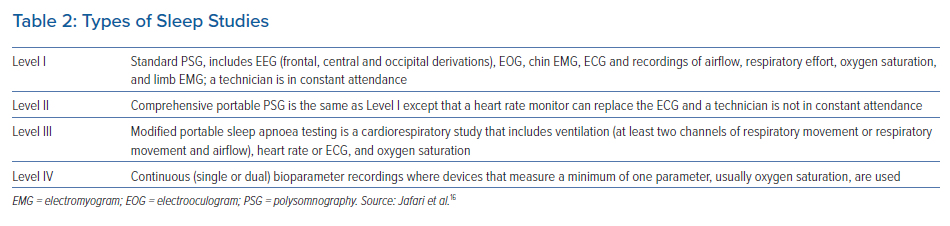
The gold standard for the diagnosis of sleep apnoea is PSG, which uses various methods to simultaneously and continuously record neurophysiological, cardiopulmonary and other physiological parameters over the course of several hours, usually during an entire night.16 There are four types of sleep studies available, depending on the number of physiological variables being recorded (Table 2). In patients with AF, at-home PSG has largely replaced in-laboratory testing given issues of patient compliance, underlying costs and insurance constraints. However, clinicians should be aware that a home sleep test (HST) may yield a false negative test result or underestimate the severity of OSA compared with in-laboratory PSG.17
PSG allows one to grade the severity of sleep apnoea based on the apnoea–hypopnoea index (AHI). An apnoea or hypopnoea event is counted if it lasts at least 10 seconds.18 Obstructive and central apnoeas are differentiated by looking at the thoracoabdominal band readings to determine whether there was persistent or absent respiratory effort. Obstructive apnoeas show movement, indicating that respiratory effort was present, whereas central apnoeas demonstrate no movement, so no respiratory effort was measured.
Conversely, hypopnoeas are more difficult to define. The current American Academy of Sleep Medicine recommended definition for hypopnoeas includes a minimum 10-second duration and a ≥30% airflow reduction associated with ≥3% oxygen desaturation and arousal.19 The acceptable definition of hypopnoeas used by the Centers for Medicare & Medicaid Services is a minimum 10 s duration and ≥30% airflow reduction associated with ≥4% oxygen desaturation; there is no requirement to demonstrate arousal.20 Based on the AHI, patients are categorised as having no OSA (AHI <5), mild OSA (5 ≤ AHI < 15), moderate OSA (15 ≤ AHI <30) or severe OSA (AHI ≥30).
Alternative methods to at-home PSG have been proposed. In recent years, there has been increased use of WatchPAT (PAT = peripheral arterial tonometry) as a method of home sleep testing. The system is grounded on a wrist-worn device with a finger probe that obtains PAT signals and oxygen saturation levels, a snoring and body position sensor that is positioned under the sternal notch and an accelerometer that is embedded in the wrist unit.21 The device’s algorithm detects respiratory (apnoea/hypopnoea) events and sleep–wake status, and determines sleep stages. The ability to differentiate sleep stages is an important improvement over more conventional methods of home sleep testing.22,23 A recent study assessed the simultaneous use of a WatchPAT device in AF patients undergoing in-laboratory PSG, finding very high correlation between the two.21
The diagnostic utility of overnight oximetry in AF patients has been evaluated as a means to provide a more accessible and cost-effective tool for the diagnosis of sleep disordered breathing (SDB). Among 439 patients with AF, overnight oximetry was used in combination with a novel automated computer algorithm to calculate the oxygen desaturation index (ODI; number of desaturations/hour).24 The ODI scores for these patients were subsequently compared to AHI scores obtained from an in-laboratory PSG. An ODI cut-off of 4.1 events/h had 91% sensitivity and 83% specificity for identifying patients with an AHI ≥15; similarly, an ODI cut-off of 7.6 events/hour had 89% sensitivity and 83% specificity for identifying patients with an AHI ≥30.24 Conversely, the negative predictive value for exclusion of OSA by oximetry was >95%.24 Thus, it may be possible to use overnight oximetry-derived ODI as a low-cost, accessible screening tool for SDB, especially as a method of excluding moderate–severe sleep apnoea.
Regardless of the method chosen for diagnosis, additional limitations must be recognised. The severity of OSA is primarily determined by the AHI, which is the number of hypopnoeas and apnoeas per hour of sleep. However, the detrimental effect of sleep apnoeas on the cardiovascular system may depend on the sleep stage in which the apnoeas occur, as well as whether they are associated with autonomic activation and cortisol arousals. For example, rapid eye movement (REM) sleep is associated with greater sympathetic activity, decreased vagal tone and cardiovascular instability. Consequently, obstructive respiratory events occurring during REM sleep could yield worse cardiovascular outcomes than events occurring in non-REM sleep.25 Furthermore, AHI scores do not fully address the absolute degree and duration of oxygen desaturation, and thus do not distinguish between patients with shorter, more mild episodes of OSA and those who have longer episodes with a greater degree of oxygen desaturation.25
In addition to making sleep testing more readily accessible to patients, other issues with sleep testing must be addressed. The VARIOSA-AF Study used data from implanted pacemakers to demonstrate that there is day-to-day variability in the degree of SDB in AF patients.26 That observational cohort study evaluated 72 patients with a pacemaker capable of measuring a respiratory disturbance index (RDI), with an RDI ≥20/hour considered a marker of severe SDB. Individual patients showed a significant variability in nightly RDI (absolute SD of ±6.3 events/hour; range 2–14 events/hour). The nights with the highest RDI were associated with a higher likelihood of experiencing >5 minutes, >1 hour or >12 hours of AF on the same day.26 These data suggest that symptomatic patients who test negative for OSA may benefit from repeated testing due to the day-to-day variability in respiratory events.
Treatment of Obstructive Sleep Apnoea in AF Patients
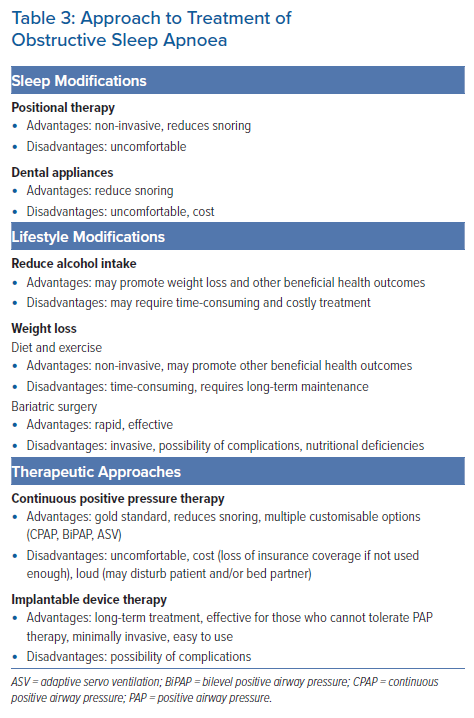
There has been great interest in treating OSA in patients with AF. Treatment has focused on modifications during sleep, lifestyle modifications, institution of positive airway pressure (PAP) and implantable device therapy (Table 3). Current guidelines state that optimal management of OSA may be considered to reduce AF incidence, AF progression, AF recurrences and symptoms.27 However, this carries only a Class IIb level of recommendation with Level C evidence, suggesting weakness in the currently available data. Until randomised clinical trials can be performed, it is unlikely that guidelines will impart a higher level of recommendation for treatment of OSA in AF patients.
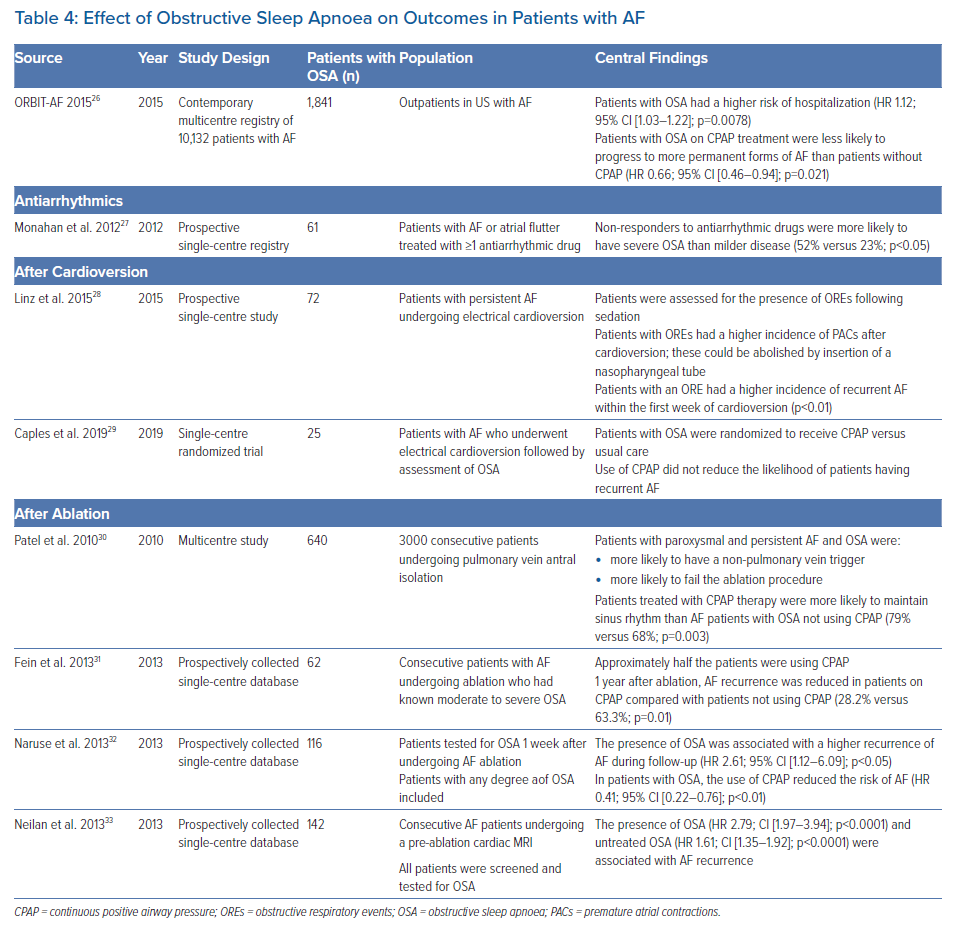
Numerous studies have evaluated the consequences of OSA in AF patients, as well as the impact of continuous positive airway pressure (CPAP) on outcomes (Table 4). The ORBIT-AF, a registry of outpatients in the US with AF, enrolled 10,132 patients, 18% of whom had clinician-defined OSA.28 In adjusted analyses, patients with OSA had a higher risk of hospitalisation (HR 1.12; 95% CI [1.03–1.22]; p=0.0078), but no difference in the risk of death (HR 0.94; 95% CI [0.77–1.15]; p=0.54), the composite of cardiovascular death, MI and stroke/transient ischemic attack (HR 1.07; 95% CI [0.85–1.34]; p=0.57), major bleeding (HR 1.18; 95% CI [0.96–1.46]; p=0.11) or AF progression (HR 1.06; 95% CI [0.89–1.28]; p=0.51).28 However, patients with OSA on CPAP treatment were less likely to progress to more permanent forms of AF than patients without CPAP (HR 0.66; 95% CI [0.46–0.94]; p=0.021).28 A limitation of that study is that the results of sleep testing were not available and so the severity of underlying OSA was unknown.
The severity of OSA can influence the response to antiarrhythmic drug (AAD) therapy. One study evaluated 61 patients treated with an AAD for symptomatic AF who underwent overnight PSG.29 Twenty-four (40%) of those patients had severe OSA. Ultimately, 30 (49%) patients were rhythm controlled with an AAD, defined as a patient remaining on the same AAD therapy for ≥6 months with a ≥75% reduction in symptomatic AF burden.29 Non-responders to an AAD were more likely to have severe OSA than milder disease (52% versus 23%; p<0.05); those with severe OSA were less likely to respond to an AAD than participants with non-severe OSA (39% versus 70%; p=0.02).29
This issue has also been examined in a population of AF patients undergoing electrical cardioversion. Linz et al. examined 72 patients with symptomatic persistent AF and an ESS score >10 who were undergoing cardioversion.30 Two minutes after cardioversion, which was performed under deep sedation, obstructive respiratory events (OREs) were determined visually and defined as cessation of air flow with maintained breathing efforts for at least 10 seconds. An ORE was observed in 40 (56%) patients; the incidence of premature atrial contractions (PACs) following cardioversion was significantly higher in patients with than without OREs (mean [± SD] 7 ± 2 versus 1 ± 1 /10 seconds, respectively; p<0.01). In addition, upon insertion of a nasopharyngeal tube, the occurrence of PACs was reduced by 79%.29 Moreover, of 20 patients who were found to have recurrent AF 1 week after cardioversion, 16 were in the ORE group and only four patients were among those without OREs (p<0.01).30 A small study randomised 25 patients diagnosed with OSA following cardioversion to receive CPAP therapy or usual care.31 In that study, the use of CPAP did not reduce the likelihood of patients having recurrent AF.
One of the major unanswered questions is the effect of OSA, and CPAP therapy, on outcomes after catheter ablation of AF. The largest study to date evaluated 3,000 patients enrolled in a multicentre database, 640 (21%) patients having at least moderate OSA.32 No information was provided on how OSA was assessed or when it was assessed relative to ablation. The ablation included pulmonary vein antral, posterior wall and superior vena cava isolation in patients with paroxysmal AF; in persistent AF patients, additional lesions were delivered on the left-side of the septum and defragmentation of both atria was performed. Finally, high-dose isoproterenol was infused to elicit non-pulmonary vein triggers. AF patients with OSA were more likely to have a non-pulmonary vein trigger and more likely to fail the ablation procedure. Patients with OSA treated with CPAP were more likely to maintain sinus rhythm (79% versus 68% in patients not using CPAP; p=0.003).32 However, compliance with CPAP was self-reported.
Subsequently, a number of prospectively collected single-centre database studies have been reported. Fein et al. assessed a database of 426 patients who underwent AF ablation; of these, 62 (15%) patients had PSG-confirmed moderate or severe OSA.33 CPAP use was present in 32 (52%) patients with OSA and resulted in a higher AF-free survival rate (71.9% versus 36.7%; p=0.01) and AF-free survival off an AAD or repeat ablation following pulmonary vein isolation (65.6% versus 33.3%; p=0.02). The AF recurrence rate in CPAP-treated patients was similar to that in patients without OSA (HR 0.7, p=0.46).33 Naruse et al. evaluated 153 patients who underwent a PSG 1 week after AF ablation.34 An AHI ≥5 was present in 116 (76%) patients. The presence of OSA was associated with a higher recurrence rate of AF during follow-up (HR 2.61; 95% CI [1.12–6.09]; p<0.05). CPAP was used in 82 (71%) patients with OSA and was associated with a reduced risk of AF (HR 0.41; 95% CI [0.22–0.76]; p<0.01).34 Finally, Neilan et al. found OSA was present in 20% of 720 patients who underwent a cardiac MRI prior to AF ablation.35 The presence of OSA (HR 2.79; 95% CI [1.97–3.94]; p<0.0001) and untreated OSA (HR 1.61; 95% CI [1.35–1.92]; p<0.0001) were both associated with AF recurrence.
In contrast to these studies, others have found that treatment of sleep apnoea does not affect outcomes. A meta-analysis of PAP therapy in patients with sleep apnoea included 10 randomised clinical trials (nine CPAP; one adaptive servo ventilation).36 Combined, the trials included 7,266 patients; of these, 356 (5%) patients experienced a major adverse cardiovascular event (MACE) and 613 (8%) died. The use of PAP did not reduce the risk of MACE, death due to a cardiovascular event or all-cause death. The same was true for acute coronary syndrome, stroke and heart failure. Furthermore, there was no association between PAP for different levels of apnoea severity, follow-up duration or adherence to therapy.36 This highlights the need for caution in misinterpreting the benefits of PAP therapy based solely on observational data.
Our Approach
The 2020 ESC AF guidelines emphasise the importance of a patient-centred, integrated care model in patients with AF. Such an approach provides patients with the tools needed for lifestyle modification, adherence to treatment protocols, education and psychosocial management. These tools are best provided via a multidisciplinary strategy that focuses on minimising the effects of comorbidities and risk factors on AF, reducing stroke risk and controlling rate and rhythm while adhering to patients’ preferences. These are the guiding principles for an AF centre of excellence. These centres recognise that successful AF treatment requires many strategies, which are often outside the expertise of any one clinician, and they use a team-based approach that refers and manages patients by ensuring consistent follow-up and effective communication between clinicians and patients in a methodological manner.10
At the Valley Health System, we created the Snyder Center for Comprehensive Atrial Fibrillation, which provides evidence-based, patient-centred and team-directed health care to our AF patients. Services outside of those provided by electrophysiologists include nutrition, weight loss management, fitness programs, evaluation and management of sleep apnoea and educational and behavioural-based patient seminars. With respect to sleep apnoea, all AF patients are screened using the ESS and STOP-BANG questionnaires, which are formally incorporated into the patient’s electronic medical record. This approach has previously been recommended by Desteghe et al.37 At-risk patients are immediately scheduled for an HST; systems have been created to ensure interpretation and evaluation of the patient by a sleep specialist within 2 weeks of completion of the test. In the coming year, we aim to dispense the tests directly from our office at the time of the patient’s clinical evaluation to further streamline the process.
Patients with sleep apnoea who begin therapy are remotely monitored monthly by a research coordinator for compliance. Patients using therapy <70% of the time are flagged for the electrophysiologist; a patient coordinator initiates a conversation with the patient to understand the reason for non-compliance. If needed, some patients are asked to pursue alternative therapeutic options, such as implantable device therapy or even bariatric surgery. Finally, we prospectively monitor our patients for AF events to understand the relationship between sleep apnoea, treatment and AF events.
To summarise, a definitive understanding of this topic would require consideration of the following:
- ensuring all patients with AF undergo an HST;
- repeating the HST to understand the variability in the presence and severity of sleep apnoea;
- determining what severity of sleep apnoea (any, only moderate or severe) warrants consideration of CPAP therapy;
- determining whether CPAP must be started before an intervention (e.g. cardioversion, ablation) or can be started after the intervention; in either case, a randomised, multicentre assessment of CPAP therapy is warranted;
- having an objective mechanism in place to assess compliance with CPAP;
- incorporating a standardised ablation protocol; and
- using an objective assessment of AF recurrence (e.g. implantable loop recorder).
Conclusion
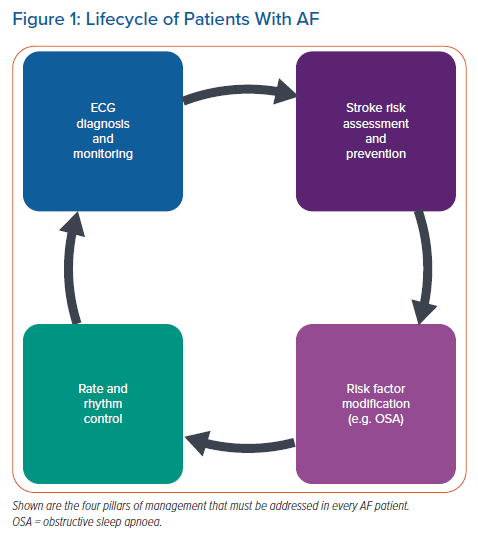
In the lifecycle of the AF patient, risk factor modification has become an important pillar of management (Figure 1). To that end, OSA has emerged as an important modifiable risk factor in AF patients. Nonetheless, major challenges remain to the effective interpretation of the existing literature. Thus, a multicentre randomised clinical trial of CPAP therapy in AF patients is very much needed in order to inform clinical practice. Such a study is needed to determine the precise magnitude of benefit (especially as it relates to AF) and, importantly, to exclude the potential for harm.
Clinical Perspective
- The likelihood is that all patients with AF should be screened for OSA.
- Although screening questionnaires are typically used, some form of sleep testing should be used.
- In the laboratory, polysomnography is the gold standard; however, home sleep testing is the most practical approach in clinical practice. Various techniques are used, and each has its inherent advantages and disadvantages.
- Largely observational studies support the positive impact of CPAP therapy on outcomes after treatment in AF patients.
- There is a major need for randomised clinical trials to assess the impact of CPAP therapy on arrhythmia and other outcomes in AF patients.