The prevention of sudden cardiac death is one of the main goals of cardiac device therapy.1–3 ICDs are effective in sensing and treating deadly ventricular arrhythmias through complex iteratively developed rhythm identification algorithms.4,5 For decades, the only available implantable options proven to be effective have been transvenous ICDs (TV-ICDs) and surgical implantation of patches and epicardial leads on the heart. However, the short- and long-term risks involved with implantation are significant. The subcutaneous ICD (S-ICD) has been developed as an alternative to these transvenous devices.6 S-ICDs avoid many of the short-term risks associated with de novo implantation, such as pneumothorax or cardiac perforation, and long-term risks, such as systemic infection.6–8
For more than a decade, S-ICDs have been studied in multicentre clinical trials and have proven to be effective.7,8 Moreover, recent data suggest that S-ICDs may even be superior to TV-ICDs in some respects.9,10 Nevertheless, S-ICDs present a unique set of potential complications and risks – as well as the risks that are common to both types of ICDs.8,9 Complications and risks unique to S-ICDs are discussed in further detail in the following sections. However, the main objective of this review is to discuss the most recent literature and contemporary populations studied with this device, with a focus on the risk of inappropriate shocks (IAS) – an issue mutual to both S-ICDs and TV-ICDs.
Inappropriate Shocks
The rate of IAS in TV-ICDs with contemporary programming is typically less than 5% annually.4,5,11 Common causes of IAS in people with TV-ICDs are misdiagnosed AF or abnormal sensing in the setting of lead malfunction.9,11 In contrast, with S-ICDs the most common causes of IAS are T wave oversensing and myopotentials.12–14 Misclassification of supraventricular arrhythmias is infrequent with S-ICDs. These different aetiologies of IAS largely offset each other.15 Nonetheless – regardless of the type of implantable device – IASs are painful, hazardous and can result in psychological sequalae.11,16–18
TV-ICD sensing was developed as a beat-by-beat counter to classify arrhythmias rapidly and deliver therapy.11 S-ICD algorithms sense somewhat differently and intended to be a rhythm detector.12 This device has a more detailed morphology matching process and a much longer time to classify the rhythm.19,20 The algorithm is comprised of three phases. Phase 1 is the sensed event detection phase, which filters signals and adjusts sensitivity based on preceding QRS complexes before certifying an elevated heart rate to reduce R wave double counting and T wave oversensing. Phase 2 classifies sensed events as certified QRS complexes or as suspected oversensing events and calculates the heart rate. This includes advanced waveform algorithms that use frequency and slew-rate analysis to reject myopotentials and electromagnetic interference. Phase 3 is the decision phase during which ventricular arrhythmias are discriminated from supraventricular tachycardias.21–23
Except for the initial generation of ICDs, TV-ICDs have the pacing capabilities to terminate ventricular tachycardia without a shock. This made the programming of multiple zones important.11,24 S-ICDs only deliver full energy shocks but have two programmable zones: a conditional zone, where the discrimination algorithms are active; and a shock zone, which delivers therapy based solely on rate.20 Initially, many implanters did not activate the conditional zone for programming as the same therapy is delivered in both zones. However, the importance of the conditional zone for discrimination became clear in analyses of prospective clinical trials, so it is now standard.25
Advances have been made in the programming and algorithms of both types of devices to reduce IAS rates. Ironically, this was because TV-ICDs were classifying rhythms and delivering therapies too quickly. Clinical trials showed that prolonging detection in TV-ICDs reduced IAS.4,11 In contrast, the duration of detection is not programmable in the S-ICD. However, improvements in the SMART Pass technology reduced IAS rates by 50% in real-world studies.26,27
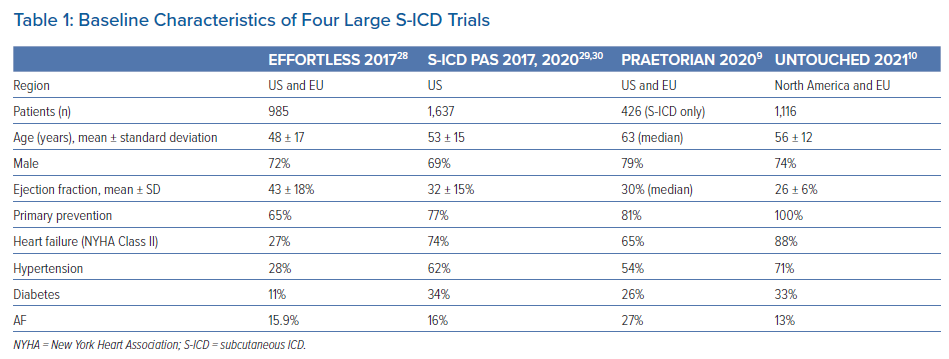
Previous cohorts that studied outcomes in S-ICD, such as the EFFORTLESS registry and S-ICD IDE studies, enrolled patients that were younger and with fewer comorbidities and demonstrated higher IAS rates.7,28 The Food and Drug Administration mandated a post-marketing registry of the S-ICD (Post-Approval Study) to include more typical ICD patients.29 Early results from this planned 5-year registry showed that the device performed well despite a sicker cohort of patients.30
UNTOUCHED
The Understanding Outcomes with the S-ICD in Primary Prevention Patients with Low Ejection Fraction (UNTOUCHED) was designed as a multinational, prospective trial to investigate limitations of S-ICDs in a higher risk population of patients.10 The trial spanned almost 3 years across North America and Europe, enrolling more than 1,100 patients with left ventricular ejection fractions ≤35% due to both ischaemic and non-ischaemic aetiologies. Patients who had indications for pacing or cardiac resynchronisation therapy, history of sustained ventricular arrhythmias, New York Heart Association classification IV and life expectancy shorter than 18 months were excluded from the study. Patients underwent standard pre-implant screening and devices were programmed based on MADIT-RIT TV-ICD programming to optimise detection and appropriate arrhythmia therapy.11 The primary endpoint for the study was the IAS-free rate at 18 months, which was compared to a performance goal of 91.6% (MADIT RIT arms B [higher rate] and C [longer duration to therapy], which is the standard for contemporary programming of TV-ICDs). An important feature of the study design was the use of prescriptive programming requiring a conditional zone at 200 BPM and shock zone at 250 BPM.10
Approximately 87% of patients had more than one passing vector in the supine and upright position at screening, and adherence to prescribed programming was approximately 98% at hospital discharge and 96% throughout the study. IAS due to cardiac oversensing occurred in 2.7% of patients, with the most common cause being T wave oversensing (1.6%). Non-cardiac oversensing (including myopotentials) occurred in 1.4% of patients. Remarkably, there were no cases of supraventricular tachycardia misdiagnosis or discrimination errors. Overall, at 18 months, the IAS-free rate was 95.9%. Moreover, the complication-free rate was 95.8% at 30 days, which satisfied the performance goal of 93.8%. Despite a cohort with greater left ventricular dysfunction and heart failure, the UNTOUCHED trial outcomes demonstrated the lowest ever IAS rate compared to prior S-ICD trials and the MADIT-RIT trial.10
Table 1 shows a comparison of baseline characteristics between four major multicentre S-ICD trials. In the UNTOUCHED trial, regression analysis revealed that predictors of IAS were history of AF and two-incision implant technique.10 It is postulated that distal lead migration may result in detection of myopotentials resulting in IAS. However, in a direct comparison of three- versus two-incision technique, there were no differences in first shock efficacy during conversion testing, shock impedance, complication-free survival at 5 years, or IAS rate at 5 years.31
PRAETORIAN
The long-anticipated Prospective Randomized Comparison of Subcutaneous and Transvenous Implantable Cardioverter Defibrillator Therapy (PRAETORIAN) was a head-to-head trial comparing S-ICDs to TV-ICDs in terms of device-related complications and IASs.9 The study spanned almost seven years and included 876 patients across Europe and the US. The majority of patients were men and had ischaemic cardiomyopathy with a median left ventricular ejection fraction of 30%. Over an almost 50-month follow-up period, the incidence of IAS in a subgroup analysis was slightly higher in the S-ICD group, though not statistically significant, and were mostly due to cardiac oversensing. Notably, appropriate ICD shocks were more frequent in the S-ICD group, as the system is incapable of delivering anti-tachycardia pacing. In the TV-ICD group, the rate of anti-tachycardia pacing was higher, and successfully terminated 55% of all treated ventricular arrhythmias.9
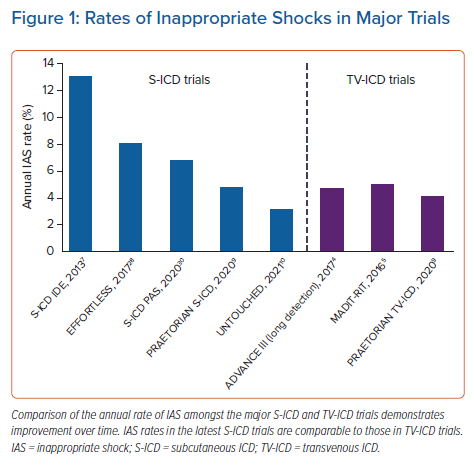
The primary endpoint of the PRAETORIAN trial was a composite of IASs and device-related complications. The S-ICD group had a nonsignificant trend towards more shocks while the TV-ICD group had a trend towards more device-related complications and significantly more lead-related complications.9 It is noteworthy that the majority of patients in this trial had older second-generation devices in which SMART Pass filter is not available or not activated automatically. In the UNTOUCHED study, a majority of patients had more contemporary third-generation devices with SMART Pass filter activated, and therefore the IAS rate was lower in this study than in either arm of the PRAETORIAN trial.10 Figure 1 provides a comparison of annual IAS rate between five S-ICD trials and three TV-ICD trials.
Efficacy
The S-ICD delivers all shocks at 80 J and has the ability to reverse vector polarity similar to TV-ICDs for unsuccessful defibrillation. Although average defibrillation thresholds of S-ICDs are threefold higher than those of TV-ICDs, 80 J shocks provide a large safety margin.20 Failure of conversion with the first shock is predicted by patient height and BMI.30 In an analysis of the S-ICD IDE population, lower BMI and shock impedance were associated with higher conversion success rates while white race was associated with lower conversion success rates.32 Various trials have reported an 83–90% success rate for first shock in TV-ICDs and 97.3–99.6% overall shock efficacy.1,32–35 S-ICDs were initially reported to have 100% sensitivity for detection of induced VF and 98% shock efficacy.6 However, a more recent multicentre study of 137 patients undergoing conversion testing at time of implantation revealed undersensing with >18 seconds time-to-therapy in 14% of patients and absence of therapy related to noise oversensing in 6% of patients.36 This finding has not been confirmed in much larger multicentre registries and time to therapy >20 seconds is well recognised in a minority of patients during testing.9,10,29,30 Whether conversion testing is still needed routinely at implantation is unclear given the extremely high success rate of such procedures in prospective studies.10,29,30
Finally, chronic conversion testing performed ≥150 days after implantation revealed a 96% success rate with 65 J shock and 100% with 80 J shock. In the same study where 119 spontaneous ventricular arrhythmia episodes were observed in 21 patients, the S-ICDs demonstrated a 92.1% first shock success rate with 100% overall conversion rate.7 In fact, the START trial had already demonstrated equivalent S-ICD efficacy in detection and discrimination of ventricular arrhythmias.20
Complications
Complications associated with TV-ICD implantation include vascular or brachial plexus injury, cardiac perforation and tamponade, pneumothorax or haemothorax, lead dislodgement or malfunction and infection and haematoma formation.37 According to a systematic review of real-world reported data from the National Cardiovascular Data Registry, TV-ICD implantation carries a 3.08% risk of complication. However, a pooled complication rate from randomised clinical trials reveals a rate of 9.1%, suggesting underestimation of long-term complications due to variable reporting.38 S-ICDs were designed, in part, as a way to circumvent many of the risks associated with TV-ICDs. Unique approaches to S-ICD implantation are needed such as the need for deep sedation or regional anaesthesia, although general anaesthesia is not obligatory.39 Moreover, anticoagulation is a risk factor for haematoma formation in these devices.40
Finally, the intermuscular technique was adopted to reduce pocket complications and infections. Although there is a learning curve associated with successful intermuscular implantation (between the latissimus dorsi and serratus anterior muscles), this technique has been shown to reduce pocket infections, haematoma formation, and demonstrate lower shock impedance and defibrillation threshold by allowing more posterior device position with less adipose tissue separating the pulse generator and the rib cage.41 In fact, combining two-incision and intermuscular technique resulted in the lowest risk PRAETORIAN scores.42
Early trials of S-ICDs demonstrated higher complications rates, partly attributable to the learning curve of implantation. In a Dutch cohort of 118 patients, 16 experienced complications with higher frequency in the initial 15 implantations.12 The S-ICD IDE study reported a 180-day complication-free rate of 92.1%.7 The EFFORTLESS Registry study reported complication-free rates of 97% and 94% at 30 and 260 days, respectively.28 Importantly, in-hospital complication rates are 0.9%, similar to TV-ICDs.43 Most notably, S-ICD infections are uniformly not associated with bacteraemia or systemic involvement as they are with TV-ICDs.44
In the PRAETORIAN trial, the incidences of composite primary end point (IAS and device-related complications) between the two systems were nearly equivalent. Device-related complications occurred in 31 patients with S-ICD and 44 with TV-ICDs. The incidence of complications within the first 30 days and lead-related complications were lower in the S-ICD group. Importantly, rate of pocket haematoma was slightly higher in the S-ICD group. These similar rates of complications and IAS between S-ICDs and TV-ICDs demonstrate the noninferiority of S-ICDs in select patients without pacing indications.9 In the UNTOUCHED trial, complication rates remained low despite a higher risk population of patients in the study.10
As of December 2020, there have been 27 reported cases of electrode body fractures (Model 3501) distal to the proximal sense ring, resulting in a cumulative occurrence rate of 0.2% at 41 months and potential for life-threatening harm of 1 in 25,000 (0.004%) at 10 years.45 However, this needs to be placed in perspective of TV-ICD lead survival rates of 85% and 60% at 5 and 8 years, respectively.46 Additionally, there is an advisory on the elevated likelihood of a low voltage capacitor (in models A209 and A219) causing accelerated battery depletion.47 The battery longevity of the S-ICD is significantly less than that of single chamber TV-ICDs.48 More long-term observation is needed to better define the magnitude and consequences of these issues along with shared decision-making regarding the optimal device to implant.
Finally, modular cardiac rhythm management systems are in development to address the deficit of anti-tachycardia pacing in S-ICDs. One such system is the EMPOWER leadless pacemaker that can be implanted at a later date if anti-tachycardia pacing is desired or indicated. This system communicates with the S-ICD system as one unit, broadening the applicability of totally extra-vascular cardiac rhythm management systems.49 The other concept is an ICD with an extravascular yet substernal lead that has shown promising results for successful pacing and defibrillation.50
Conclusion
The ICD is a cornerstone in treatment for the prevention of sudden cardiac death.2 Traditional TV-ICDs are associated with certain short-term risks such as pneumothorax, vascular and valvular injury, cardiac perforation and infection. Long-term risks include lead malfunction and systemic infection resulting in endocarditis.37,38,46 The S-ICD system was developed in order to further mitigate the potential for complications especially in higher risk patients.6 Recent S-ICD studies show favourable outcomes of this device even in patients with similar co-morbidities to typical TV-ICD cohorts.
Despite the very encouraging results from recent S-ICD trials, there are limitations and complications. First, patient selection is important, as S-ICDs do not provide pacing-therapy currently.6,7 Second, pre-procedural screening is important to determine appropriate sensing of the cardiac electrical complex to reduce the risk of undersensing or T wave oversensing.6–10,32,36 The importance of electrocardiographic screening for appropriate sensing in multiple postures has been a requirement for this device as part of labelling and has been employed in all major trials of the S-ICD. More recently, an automated screening tool was developed to facilitate this process.51 Third, IAS occur, although iterative improvements in SMART Pass filtering and contemporary programming have reduced IAS significantly while maintaining the ability to successfully diagnose and treat ventricular arrhythmias.20,25,26,36 Lastly, the implementation of specific analgesic protocols and telephone follow-up for early device-related pain enables successful same-day discharges after outpatient implantation of S-ICDs which is an area of attention in the modern era of high-value healthcare.52
S-ICDs have been studied in large randomised clinical trials and have proven to be effective and achieve excellent arrhythmia conversion.7,20 Despite a cohort with higher left ventricular dysfunction and heart failure, as shown in Table 1, the UNTOUCHED trial outcomes demonstrated the lowest ever IAS rate compared to prior S-ICD trials and the MADIT-RIT trial as depicted in Figure 1. Finally, the PRAETORIAN trial demonstrated noninferiority of S-ICDs to TV-ICDs in terms of device-related complications and IAS.9,10
Clinical Perspective
- Subcutaneous ICD technology has evolved to meet clinical standards and noninferiority in terms of device-related complications when compared to transvenous ICDs, as shown in the PRAETORIAN trial.
- The UNTOUCHED trial demonstrated that inappropriate shock rates of subcutaneous ICDs in a sicker cohort of patients are similar, if not lower, compared with transvenous ICDs.
- Appropriate patient selection and screening alongside contemporary device programming are paramount to the success of subcutaneous ICDs.