The plasticity of the heart enables it to adapt to certain pathological insults and to maintain the cardiac output necessary to satisfy the metabolic requirements of the body.1 Although beneficial at first, this process of ventricular remodelling can have detrimental effects on cardiac function and contribute to arrhythmogenesis.2 Sudden cardiac death due to ventricular tachyarrhythmias accounts for up to 60% of cardiovascular deaths.3 Despite improvements in cardiovascular risk management and therapeutic strategies for heart failure, sudden cardiac death remains an important healthcare issue.4 A considerable number of patients rely on the ICD for the prevention of sudden cardiac death.5 While ICD therapy is highly effective in ending ventricular arrhythmias, it does not prevent malignant arrhythmias from occurring.6–8 Experiencing ICD-shocks – either appropriate or inappropriate – can result in psychosocial distress for the patient and a reduced quality of life.9,10 Additional treatment, such as antiarrhythmic drugs or radio frequency ablation, are often used as adjunctive therapy to reduce the number of ICD-shocks, but both therapeutic modalities expose the patient to potentially adverse effects.11,12 This underscores the importance of finding alternative therapies that intervene to prevent the arrhythmia (and concomitant shock therapy).
Beat-to-beat variation of repolarisation (BVR) quantified as short-term variability of repolarisation (STV), has been studied extensively in relation to arrhythmogenesis in the chronic complete atrioventricular block (CAVB) dog model.13 STV has been proposed as a novel electrophysiological parameter for the monitoring of imminent ventricular arrhythmias.14,15 This review explains the influence of ventricular remodelling on BVR and how it can lead to a higher susceptibility for arrhythmias. The severity of arrhythmias is diverse; therefore, this review also explores the relation of STV to the severity of the arrhythmic outcome. It will also discuss the clinical implications of STV.
Ventricular Remodelling in the Chronic Complete Atrioventricular Block Dog Model
The CAVB dog model is a widely used animal model to study ventricular remodelling and its relation with ventricular arrhythmias.16–18 It is a model of compensated biventricular hypertrophy with a long QT phenotype and is reproducibly inducible for torsades de pointes (TdP) arrhythmias when challenged with a delayed rectifier outward potassium current (IKr)-blocker such as dofetilide.19–21 Creation of complete atrioventricular block, by means of His bundle ablation, forces the ventricles to activate from an infranodal focus of the conduction system resulting in a slow idioventricular rhythm. As a result of this bradycardia, the cardiac output drops abruptly and there is volume overload. The decrease in cardiac output and the altered asynchronous activation form insults to the heart and trigger electrical, contractile and structural ventricular remodelling as compensatory mechanisms.
Whereas structural remodelling forms slowly and reaches a stable condition between 12 and 16 weeks of CAVB, electrical and contractile remodelling are present from 2 weeks of CAVB and coincide with the inducibility of TdP arrhythmias in the CAVB dog model.13,22–28 The most striking feature of electrical remodelling is the prolongation of repolarisation time.15,18,28 On a cellular level, this is explained by the downregulation of the slow and rapid components of the delayed rectifier outward potassium currents (IKs and IKr).29 The prolonged action potential duration (APD) provides more time for the contractile process of the cardiomyocyte. In combination with altered Ca2+ handling this leads to Ca2+ overload in the CAVB dog model.30–32 In an electrically remodelled heart, the Ca2+ overload can lead to early after depolarisations (EADs) that can trigger TdP arrhythmias in this animal model.
Repolarisation Reserve is Reflected by Beat-to-beat Variation of Repolarisation
The normal cardiomyocyte possesses a redundancy in repolarising currents, enabling it to withstand internal and external challenging factors on the repolarisation, which is also called the repolarisation reserve.33 The duration of repolarisation is within normal limits and fluctuates slightly between subsequent beats. Repolarisation reserve diminishes due to electrical remodelling, causing the heart to become more susceptible to repolarisation-related ventricular arrhythmia. This phenomenon is reflected by the increased temporal dispersion of repolarisation, or BVR, which can be quantified as STV. This should not be confused with heart rate variability (HRV), which reflects the balance of the activity of the autonomic nervous system and can serve as an indicator of cardiovascular integrity and prognosis.34,35
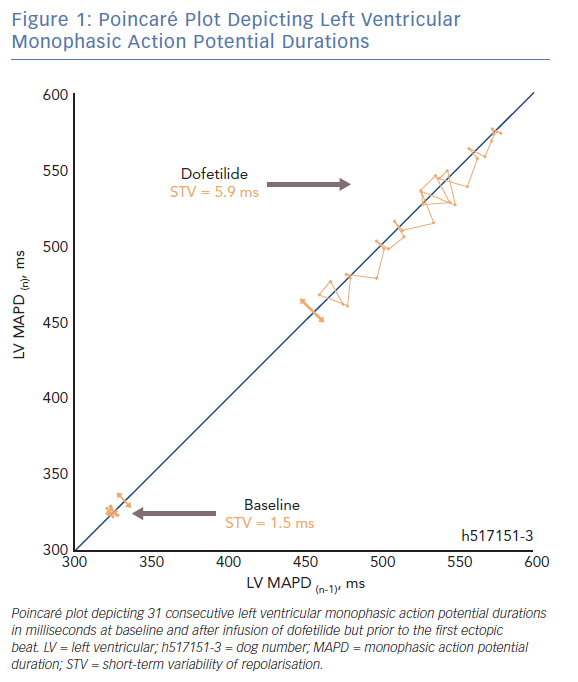
In a Poincaré plot, the repolarisation duration of a predetermined number of consecutive beats (n=31) is plotted against the duration of each previous beat (Figure 1). The difference in repolarisation between two subsequent beats is reported by each deviation from the line of identity. The average deviation of repolarisation from the line of identity for a number of beats reports STV, expressed in milliseconds (ms). STV is calculated according to the formula  , where D represents repolarisation duration and N is the total number of beats.36
, where D represents repolarisation duration and N is the total number of beats.36
Increased Temporal Dispersion of Repolarisation as a Substrate and Trigger for Torsades de Pointes
There are two fundamental requirements for ventricular arrhythmias such as TdP. The first being the need for a substrate that renders the heart susceptible to the development of arrhythmias. Electrical remodelling in the CAVB dog model diminishes the repolarisation reserve, thereby forming part of the substrate. During sinus rhythm and acute AV-block in the absence of remodelling, TdP arrhythmias cannot be induced when the animals are challenged with dofetilide.13,26 It is also evident that STV is higher at baseline in CAVB dogs where TdPs can be induced repetitively compared with resistant CAVB dogs.37,38 Sudden cardiac death can occur in the canine CAVB model and is associated with a higher STV at baseline.39 This indicates that proarrhythmic ventricular remodelling is associated with an increased STVbaseline which reflects the decreased repolarisation reserve.
The second fundamental for ventricular arrhythmias is the presence of a trigger. Temporal dispersion of repolarisation also fulfils this second role. Alongside ventricular remodelling, anaesthesia and bradycardia further challenge the repolarisation reserve in the canine CAVB model.14,21,40 When the ‘final hit’ in the form of an IKr-blocker is infused, the absolute value of STV increases abruptly in the minutes preceding a storm of TdPs (STVarrhythmic).13–15,36,41,42 Moreover, STV has been shown to be a superior repolarisation parameter to predict the imminent proarrhythmic outcome, compared with repolarisation prolongation and interventricular dispersion of repolarisation.25,40
Relation of Short-term Variability of Repolarisation to Severity of Arrhythmic Outcome
The arrhythmic outcome in the inducible CAVB dogs is diverse and can range from self-terminating TdPs to TdPs requiring defibrillation for termination. Therefore, we investigated the relation of STV to the severity of the arrhythmic outcome. Articles studying STV in relation to TdP arrhythmias upon dofetilide challenge in the CAVB dog model at our lab were considered for pooled analysis (n=28). Only original articles that reported STV of the left ventricular monophasic action potential duration (STVLV, MAPD) in idioventricular rhythm-remodelled CAVB dogs and that used a standard anaesthetic regimen were considered. The QT-interval was measured on lead II of the ECG and corrected for heart rate using the van de Water formula or the Bazett formula.43,44 Original data from the remaining 11 articles was obtained. After removing duplicates, data from 64 inducible dogs was pooled.
Definition of Inducibility and Quantification of Arrhythmia Severity
A decreased repolarisation reserve enables the occurrence of single ectopic beats (sEB), multiple ectopic beats (mEB), and TdPs. A TdP has been defined as ≥5 consecutive EBs with a twisting QRS vector around the isoelectric line. A dog is considered to be inducible when ≥3 TdPs occur within 10 minutes after the start of infusion with an IKr-blocker such as dofetilide.41 When a TdP lasts for 10–12 seconds, defibrillation with thoracic patches is performed to restore normal heart rhythm.
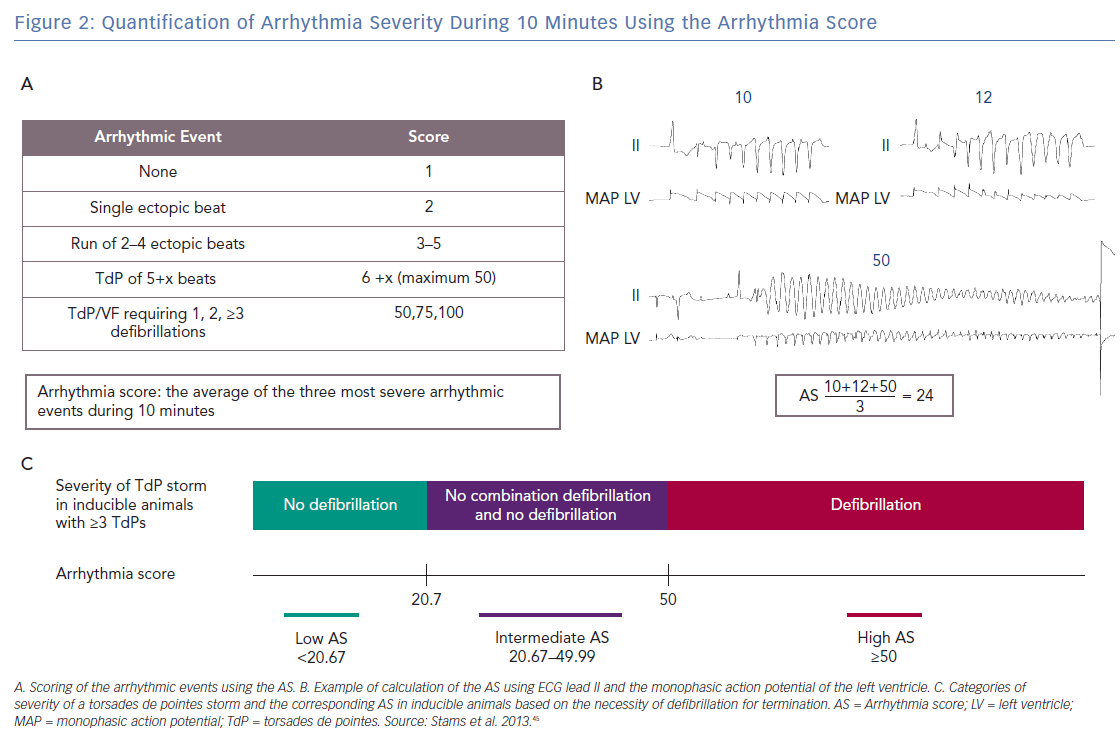
The arrhythmia score (AS) has been developed in an effort to quantify the severity of the arrhythmias.45 The AS is calculated by averaging the three most severe arrhythmias that occur during 10 minutes from the start of dofetilide infusion. Figures 2A and B show how the individual arrhythmic event is scored: 1 point is given by default for each regular beat in the absence of arrhythmic events. An ectopic beat or run of ectopic beats is scored with 1 additional point per ectopic beat to a maximum of 50. Hence, 2 points for sEB, 3–5 points for mEB, and 6–50 points for TdP. A TdP requiring defibrillation is perceived as the most severe individual arrhythmic episode in the CAVB dog model and is applied for TdPs that last ≥10 seconds and contain ≥50 beats. Therefore, defibrillation is scored with 50, 75 or 100 points depending on the number of consecutive defibrillations necessary to terminate one TdP episode (1, 2, ≥3, respectively).40,46
Figure 2C illustrates how the AS corresponds with the severity of a storm of TdPs in inducible animals with ≥3 TdPs. We consider a storm of self-terminating TdPs less severe than a storm of TdPs requiring at least one defibrillation. In an inducible animal with one defibrillated TdP (50 points), the lowest possible AS is 20.67 when the other two TdPs have the minimal duration corresponding to 6 points each

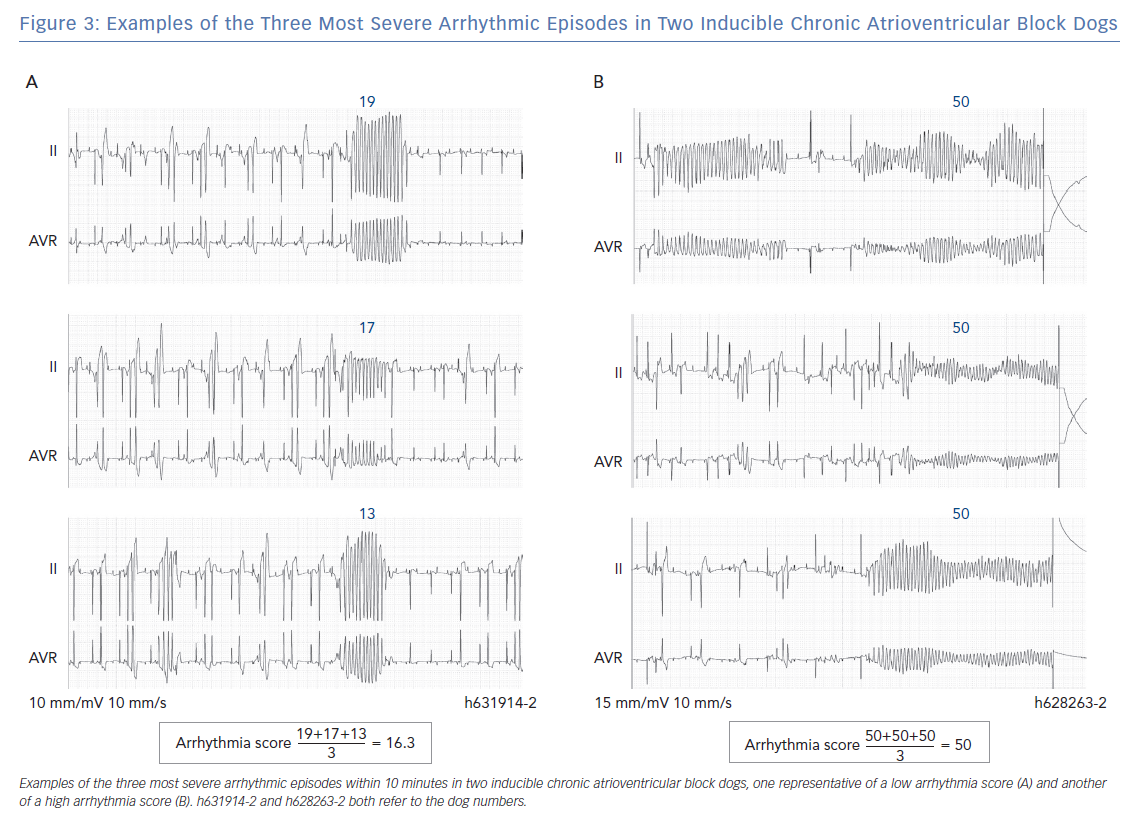
Therefore, all inducible animals with an AS ≤20.67 only have self-terminating TdPs and are part of the low AS group. An example of ECG tracings of an inducible animal with no defibrillations is shown in Figure 3A, where the AS is 16.3. The most severe storm of TdPs consists of TdPs requiring defibrillation (in the high AS group). The AS is 50, when all three most severe arrhythmic events require defibrillation (Figure 3B). However, an average of 50 points can also be obtained with three long-lasting TdPs of ≥49 beats that terminate spontaneously before 10 seconds. The AS can reach a maximum of 100 points when the three most severe arrhythmic events require ≥3 consecutive defibrillations for termination. A combination of self-terminating and defibrillated arrhythmias corresponds to an AS of ≥20.67 and <50. An example in Figure 2B shows the intermediate AS group.
STV and QTc Increase Before Torsades de Pointes
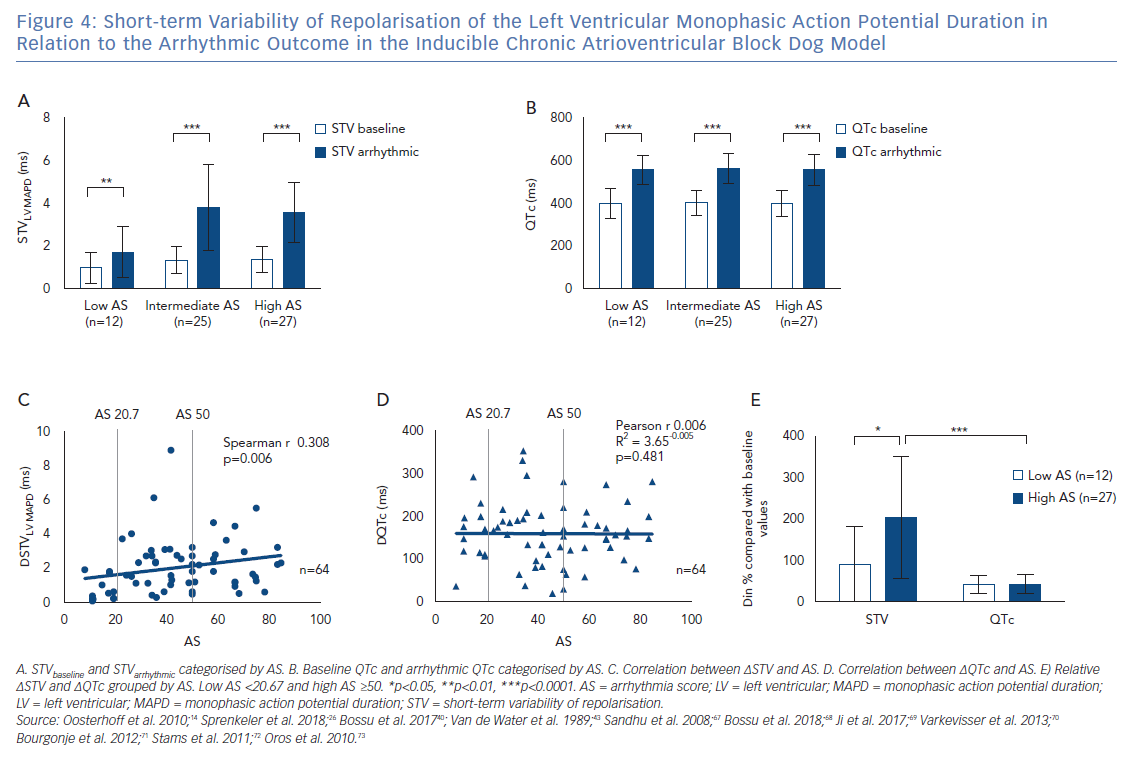
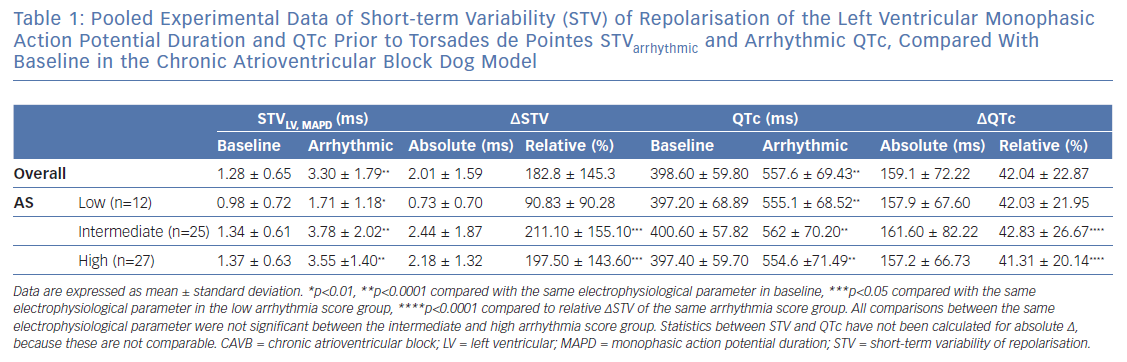
It has been described previously that STVarrhythmic is higher compared with STVbaseline in inducible CAVB dogs. As shown in Figure 4A, STVarrhythmic is significantly increased compared with STVbaseline in the three different AS groups. STV increases from 0.98±0.72 to 1.71 ± 1.18 (p=0.0020) in the low AS group, from 1.34 ± 0.61 to 3.78 ± 2.02 (p<0.0001) in the intermediate AS group, and from 1.37 ± 0.63 to 3.55 ± 1.40 (p<0.0001) in the high AS group (Table 1). STVbaseline values do not differ between the three AS groups. Whereas previous research reports that STVbaseline is significantly higher in inducible dogs compared with non-inducible dogs, STVbaseline does not seem to contribute to the severity of the arrhythmias in inducible dogs.37,38 STVarrhythmic shows a trend for higher values in the group with an intermediate and high AS compared with the low AS group, but fails to reach statistical significance. Arrhythmic QTc is also significantly increased compared with QTc baseline in the three AS groups (Figure 4B and Table 1). Neither the values of QTc baseline nor the values of arrhythmic QTc differ between AS groups.
The Rise in Short-term Variability of Repolarisation is Associated with the Severity of the Arrhythmic Outcome
Figure 4A indicates that the change from STVbaseline to STVarrhythmic (∆STVLV, MAPD) is greater in the intermediate and high AS groups compared with the low AS group. Further quantification of this observation shows a significant correlation between ∆STVLV, MAPD and the AS (Spearman r 0.308, p=0.006; Figure 4C), indicating an association between the increment of STV and the severity of the arrhythmic outcome. This correlation is absent for the change in QTc (∆QTc), whereby QTc is equally prolonged in dogs with a low AS and a high AS (Pearson r 0.006, R2 3.65-0.005, p=0.481; Figure 4D).
The AS has a heterogeneous nature, in the intermediate AS group the same AS score can be the result of a diverse combination in severity of arrhythmias. The group with an intermediate AS shows a big variation in ∆STVLV, MAPD with some outliers (Figure 2C and Figure 4C). Therefore, the relative ∆STVLV, MAPD and ∆QTc have been compared in dogs with a low AS and high AS (Figure 4E). The dogs discussed in this review with an AS score of 50 all had ≥3TdPs requiring defibrillation. Relative ∆STVLV, MAPD increases with 90.83 ± 90.28% in the low AS group and with 197.50 ± 143.60% in the high AS group (p=0.0414). This discriminative capacity is not found in the relative ∆QTc, which prolongs almost equally with 42.03 ± 21.95% in the low AS group and 41.31 ± 20.14% in the high AS group (p>0.9999). In the high AS group, the relative ∆STVLV, MAPD is significantly higher than the relative ∆QTc (p<0.0001) (Table 1, Figure 4E).
Severity of Torsades de Pointes
A higher ∆STV contributes to the severity of a storm of TdPs, but is unlikely to be determinant for the duration of an individual TdP. The mechanism of initiation of an individual TdP episode has been attributed to EAD-dependent focal activity due to reduced repolarisation reserve.47–54 The significant increase in STV before a storm of TdPs can therefore be expected. However, the mechanism underlying the perpetuation of a TdP episode is still under debate. Extensive mapping experiments have been performed in animal hearts, whereby on the one hand focal activity has been proposed as the dominant underlying mechanism.48,52–56 On the other hand, it has been suggested that nonstationary re-entry perpetuates TdPs.50,51,57 Others have observed both mechanisms to be present when TdP is perpetuated.47,49 These different observations may be due to the moment of measurement during a TdP. A TdP can deteriorate into ventricular fibrillation, which is most likely to be driven by re-entry. Therefore Boulaksil et al. clearly defined the moment of measurement, whereupon they based the conclusion that focal activity is the dominant mechanism in the perpetuation of self-terminating TdPs and during the early phase of a TdP episode, indicating triggered activity.58 Vandersickel et al. confirmed that all observed TdPs were perpetuated by focal activity in the early phase and in the case of short-lasting TdPs, but added that the longer-lasting TdP episodes were re-entry driven.59
It is important to note that the STV measured by the studies incorporated in Figure 4 preceded the first arrhythmic episode of a storm of sEB, mEB, or TdP and was correlated to the AS that was observed for 10 minutes. A higher ∆STV is therefore not associated with a longer individual episode of a TdP, but rather with the severity of the complete electrical storm of TdPs in a short period of time. The positive correlation between ∆STV and the AS can be explained in several ways. First of all, a more severely decreased repolarisation reserve reflected by a higher ∆STV, may give rise to more EADs and therefore more focal activity. The bigger amount of focal activity can in turn simply improve the odds that a proportion of the focal activity is perpetuated into a long-lasting TdP. Second, the circumstances during the early phase of a TdP may influence the perpetuation and/or degeneration into ventricular fibrillation. The increased repolarisation lability and propensity for competing foci may create a more chaotic activation pattern, and the consequences on the initiation of re-entry have not yet been explored.
One of the factors that is believed to promote the initiation of re-entry is spatial dispersion of repolarisation. However, Dunnink et al. demonstrated that spatial dispersion of repolarisation contributes to the initiation and early phase continuation of a TdP, whereby focal activity was the underlying mechanism. In mapping experiments in the CAVB dog, sEBs and the first beat of the TdP arose at the site of maximal dispersion of repolarisation and demonstrated a focal origin. The TdPs continued with a second focal beat that arose from a different location in a region of maximal repolarisation heterogeneity.56 The complex interplay between temporal and spatial dispersion of repolarisation in relation to arrhythmogenesis needs further investigation.
Clinical Implications
The observed change in STV before arrhythmia, and especially the correlation between ∆STV and severity of the imminent arrhythmic outcome, confirm the potential of STV as a possible electrophysiological marker for the monitoring of imminent ventricular arrhythmias. Data from the canine CAVB model cannot readily be extrapolated to humans, therefore clinical studies need to further investigate STVarrhythmic. In this review STV was measured with an intracardiac MAP-catheter in the left ventricle. There are also other modalities to measure STV that can be applied in humans more easily, namely the QT-interval on the non-invasive ECG and the activation recovery interval on intracardiac electrogram (EGM) in devices. The ECG has been used in descriptive studies in humans to study if STVbaseline of the QT-interval can identify the individual at risk for repolarisation-dependent ventricular arrhythmias.60–64 However, longer registrations are necessary to capture sustained ventricular arrhythmias in patients to investigate STVarrhythmic. The EGM is a promising candidate for the continuous monitoring of STV and to study STV before arrhythmia. In the CAVB dog, the STV can be reliably derived from the activation recovery interval of the EGM in the right ventricle (RV), and it increases before TdP.15 Other repolarisation parameters have been studied in patients on the RV EGM, which indicates that it is a feasible method to further investigate STVarrhythmic in patients.65–67
Conclusion
Temporal dispersion of repolarisation, quantified as STV, plays an important role in the initiation of ventricular arrhythmias like TdP. A sudden increase in STV precedes imminent TdP in the canine CAVB model. A higher increment of STV compared with baseline is associated with a more severe arrhythmic outcome, in contrast to QTc. These findings confirm the potential of STV for the monitoring of imminent pro-arrhythmic risk of the ventricles and create new options for interventions.
Clinical Perspective
- Beat-to-beat variation of repolarisation quantified as short-term variability (STV) of repolarisation reflects the diminished repolarisation reserve of the heart.
- The extent of the increase in STV before ventricular arrhythmia is correlated to the severity of the arrhythmia in the complete chronic AV-block dog model, in contrast to the change in QTc.
- STV is a promising electrophysiological parameter for the monitoring of imminent proarrhythmic risk, further clinical studies are needed to confirm this potential.