The best clinical approach to managing asymptomatic patients with ventricular pre-excitation has yet to be established. The clinical benefit of identifying and treating asymptomatic patients at risk of sudden cardiac death (SCD) has been debated since catheter ablation became effective and safe for the treatment of accessory pathways (APs). Data supporting current recommendations are mostly derived from observational studies, with few data coming from randomised clinical trials, and most studies only include a small number of patients. The very low incidence of SCD in asymptomatic patients reported by most studies has also contributed to the debate. As most patients are healthy young people who are expected to have a long life, a large, long-term randomised clinical trial evaluating mortality (no treatment versus catheter ablation) could provide the necessary evidence-based information to support recommendations. The impact that the low risk of SCD in asymptomatic patients with a chronic heart abnormality has on quality of life, career options and patients’ work life could also be considered. Evidence-based medicine has formed the basis of teaching and clinical practice for >20 years,1 but real evidence-based medicine has recently been proposed as a different approach to clinical decision-making based on expert judgment and sharing decisions with patients through meaningful conversations.2 This approach, taking into account the limitations of available evidence, could provide an alternative strategy for managing asymptomatic patients with ventricular pre-excitation.
Risk of Sudden Cardiac Death
The prevalence of Wolff–Parkinson–White (WPW) syndrome in the general population has been estimated to be one to three per 1,000 individuals3–9 and 5.5 per 1,000 among the first-degree relatives of an index case.10 However, a recent nationwide retrospective study showed a lower general prevalence of 0.36 per 1,000 in the general population aged <50 years.11 Sixty per cent of asymptomatic patients with ventricular pre-excitation are estimated to be adolescents and 40 % are thought to be individuals >30 years.12–16
Anterograde conduction through the AP disappears in 40 % of patients in the first year of life12 and in a similar percentage of cases supraventricular tachycardia (SVT) becomes non-inducible, suggesting loss of retrograde conduction.17 In children and adolescents, the probability of losing pre-excitation varies from 0 to 26 %,18–20 while 13–30 % of adults lose anterograde conduction during 5-year follow-up.21,22 The rate of spontaneous arrhythmia observed during the follow-up of asymptomatic patients ranges from 8 to 21 %.15,23–26 Studies of people with WPW syndrome have found that atrial fibrillation (AF) develops in 15 % of adults and children followed up for 10 years.27,28
The assumed mechanism of SCD and ventricular fibrillation (VF) is rapid stimulation of the ventricles due to AF rapidly conducted through the AP.29 The incidence of SCD in WPW syndrome is reported to be between 0 % and 0.6 % per year.15,24,30,31 In symptomatic patients, the risk is 3–4 % over a lifetime (approximately 0.25 % per year);15,30 higher than in asymptomatic patients.32 Although most asymptomatic patients with pre-excitation have a good prognosis, there is also a lifetime risk of malignant arrhythmias and SCD, estimated to be 0.1 % per patient year.23,33–35 More worrisome is the fact that this event can be the first manifestation of the disease in up to 53 % of patients.12,29,36,37
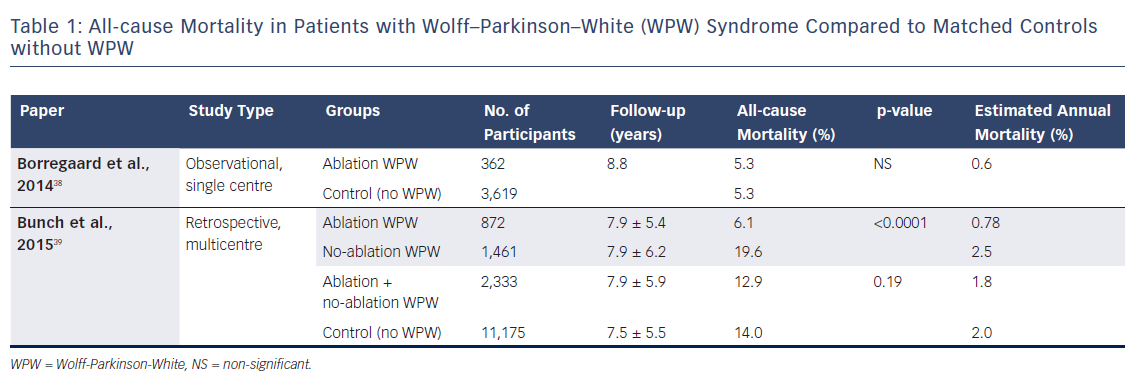
Table 1 shows all-cause mortality in patients with WPW syndrome compared with matched individuals without the condition. Borregaard et al. found no difference between patients with WPW syndrome who underwent radiofrequency ablation when compared to an age- and gender-matched control group.38 However, different results were found by a recent multicentre system-wide retrospective study comparing the long-term results in three groups of patients: those with WPW who underwent ablation; those with WPW who did not undergo ablation; and age- and gender-matched individuals without WPW.39 The patients who underwent ablation were younger (39.2 ± 17.0 years) than those who did not (46.4 ± 19.6 years) and those in the control group (43.4 ± 19.0 years). They were less likely to have hypertension, diabetes, renal failure and coronary artery disease. The total mortality and cardiac arrest rates were similar between WPW patients (ablation and no ablation) and the control group. However, cardiac death was significantly higher in the WPW population than the control group (7 % versus 4.6 %, respectively; p<0.0001). The WPW patients who did not undergo ablation had a higher risk of death in the long-term, with a trend towards more cardiac arrests and cardiac deaths than patients who did receive ablation. In the propensity analysis, the ablated group had significantly lower mortality and cardiac death rates than the non-ablated group (6.1 % versus 11.6 % and 2.8 % versus 5.9 %, respectively). A large nationwide retrospective observational study including 6,086 WPW patients aged <50 years (41.5 % ablated) showed a low mortality of 0.69 % at 11 years.11 When catheter ablation was included in the risk analysis, this variable was associated with a significantly lower risk of mortality.
A recent meta-analysis of 20 clinical studies40 showed an incidence of 0.85 SCD events (SCD/aborted or SCD/ventricular fibrillation) per 1,000 person-years, with individual rates ranging from 0.7 to 4.5 per 1,000 person-years. Although the incidence was higher in paediatric than adult patients (1.93 versus 0.86 per 1,000 person-years), this difference was not statistically significant (p=0.07). Interestingly, the risk of events was significantly higher in Italian studies (2.16 per 1,000 person-years) than in non-Italian studies (0.14 per 1,000 person-years) (p=0.008).40
Risk Factors
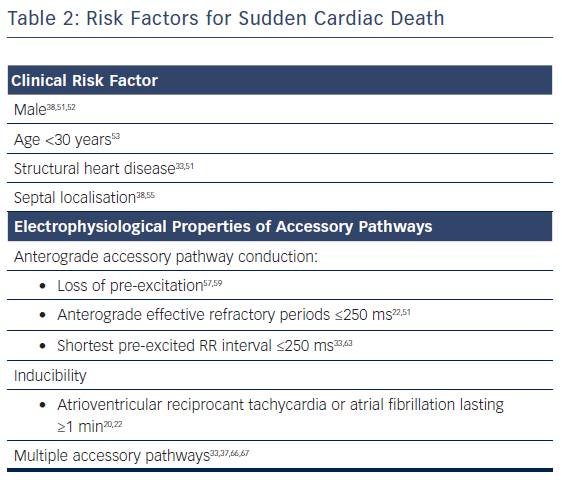
Some clinical variables such as gender, younger age, structural heart disease and septal localisation of AP have been associated with a higher risk of SCD.
Gender
Timmermans et al. demonstrated that male gender was associated with a significantly higher rate of events than female gender (13 out of 15 events, p=0.04).37 Montoya et al. observed that male gender was significantly more prevalent in patients with VF than the control group (87 % versus 67 %, p≤0.05).41 Added to this, the risk of SCD in female patients was markedly lower than in male patients in a recent meta-analysis.40
Age
Fan et al. showed that some electrophysiological (EP) properties of the AP were associated with a higher risk of SCD. A shorter anterograde effective refractory period (AERP) of the bypass tract and pre-excited R-R interval during AF were significantly less prevalent in the older group (>50 years versus <30 years).42 However, in a group of 92 asymptomatic patients (10–69 years), Brembilla-Perrot et al. demonstrated that although STV was not inducible by trans-oesophageal stimulation in any patients aged 50–69 years, rapid conduction was seen throughout the AP in all groups (21 % in 10–69 year olds: 27 % in 20–29 year olds, 27 % in 30–39 year olds, 6 % in 40–49 year olds and 23 % in 50–69 year olds).43 They concluded that older patients remain at risk of threatening arrhythmias.
Physiology
Structural heart disease is more frequent in patients with VF than those without VF in some32,41 but not all studies.44 Septal localisation (left posteroseptal, midseptal, anteroseptal and right posteroseptal) is significantly more frequent in patients with VF when compared with individuals with no VF.37 However, Klein et al. demonstrated that the localisation was no different between these groups.32 More recently, Pappone et al. found that a posteroseptal AP was present in 85 % of asymptomatic patients who experience VF (11/13) as a unique AP (7/11) or multiple APs (4/11).44
Electrophysiological Properties of AP
As clinical variables have a modest power to identify patients at high risk of SCD, risk stratification has focused on the EP properties of the AP, see Table 2. The following characteristics have been related to the risk of developing AF with rapid conduction to the ventricles:
- the ability to conduct anterogradely at very short intervals (≤ 250 milliseconds);
- the ability to sustain an atrioventricular reciprocant tachycardia (AVRT) for >1 minute (inducibility). This arrhythmia is the mechanism of AF initiation in most of patients with WPW syndrome; and
- multiple APs.
Anterograde AP Conduction at Short Intervals
Clinical and EP evaluations have been used to determine the anterograde AP conduction:
- spontaneous exercise- or drug-induced loss of pre-excitation;
- AERP by programmed atrial stimulation (transvenous or trans-oesophageal EP study);
- the shortest cycle length with one-to-one conduction by incremental atrial stimulation (transvenous or trans-oesophageal EP study); and
- the shortest pre-excited R-R interval (SPERRI) during spontaneous or induced AF.
Loss of pre-excitation observed spontaneously (intermittent pre-excitation) during an electrocardiogram (ECG) or electrocardiographic monitoring (e.g. Holter) can be observed in up to 67 % of patients45 and is considered a predictor of poor anterograde conduction and low risk of SCD.46 However, although rare, cardiac arrest in patients with intermittent pre-excitation has been observed.25 Kiger et al. recently found that intermittent pre-excitation was present in 23.4 % of paediatric patients aged 1–18 years (13.2 % on baseline ECG and 10.2 % on exercise testing or Holter monitoring). They also found an absence of statistically significant differences in the prevalence of high-risk APs (AERP, block cycle length or SPERRI during AF of ≤250 milliseconds) between patients with intermittent and persistent pre-excitation. They concluded that intermittent pre-excitation in children cannot be considered a low-risk marker according to EP criteria.47
The abrupt and complete loss of pre-excitation seen during exercise testing has been demonstrated to correlate with a long AERP.48 Two recent studies have evaluated exercise testing in 152 children with pre-excitation.49,50 Although the sudden loss of ventricular pre-excitation during exercise had a specificity and positive predictive value of 100 %, its sensitivity was low (17.7 %). Consequently, most patients who undergo an exercise stress test will need complementary risk stratification. Furthermore, although infrequent, the sudden disappearance of pre-excitation during exercise was observed in a patient with a SPERRI of 180 milliseconds.51 On the other hand, sometimes the exercise stress test shows intermittent pre-excitation at rest and persistent pre-excitation during exercise, making risk stratification confusing.52
Sodium channel-blocking agents (procainamide, propafenone and ajmaline) have been used to determine the anterograde AP conduction. Loss of pre-excitation under these drugs has been correlated with a longer AERP.51,53,54 However, these pharmacological tests are now rarely used.
An AERP ≤250 milliseconds is a frequent finding in patients with documented VF. A significantly shorter AERP was observed in 22 patients with VF (229 ± 51 milliseconds) compared to 100 patients without VF (280 ± 67 milliseconds).41 It was also observed in all three asymptomatic patients in a study who experienced VF during a mean follow-up of 37.7 months.22 Although most patients with VF have an AERP <250 milliseconds, the clinical utility of the AERP is limited as there is overlap between patients with and without VF.32 Sharma et al. observed an AERP of >250 milliseconds in 55 % of WPW patients with VF (5/9).55 Another potential limitation is the impossibility of measuring AERP because anterograde AP conduction frequently persists up to the atrial refractory period. Timmermans et al. were only able to measure the AERP in five out of 15 patients with VF.37
The shortest cycle length with one-to-one conduction by incremental atrial stimulation has been used to differentiate groups of patients with and without VF. Although all patients in the VF group were capable of anterograde conduction over the accessory pathway at cycle lengths of ≤300 ms, considerable overlap between groups was observed by Klein et al.32 Another possible limitation is the fact that sometimes it is not possible to evaluate this measurement because of the appearance of arrhythmias during incremental atrial pacing.
Probably the most useful finding in risk stratification is SPERRI during AF. In a group of 25 patients with documented VF, the SPERRI was significantly shorter than in the 73 individuals in the control group (240 ± 63 milliseconds versus 180 ± 29 milliseconds) and did not exceed 250 milliseconds in any patient in the VF group.32 Sharma et al. found a mean SPERRI of 176 ± 33 milliseconds in WPW patients with VF and a SPERRI of ≤250 milliseconds in 78 % of these patients (7/9) compared to 52 % of those without VF (30/58).55
Inducibility
In 2003, Pappone et al. published data from 212 asymptomatic patients with ventricular pre-excitation (mean age 33.6 ± 14 years, 64.8 % male) evaluated with a baseline EP study.22 At the end of a mean follow-up of 37.7 ± 16.1 months (range 14–60 months), 15.6 % of the group (33/212) had developed arrhythmic events. Of the patients that completed the follow-up EP study after 5 years, 29 % (47/162) were inducible, 10.5 % had non-sustained AF (2.5 % with isoproterenol), 12 % had sustained AVRT (5.5 % with isoproterenol) and the remaining 6.5 % had AVRT that degenerated into totally pre-excited sustained AF. Of the 20 % of patients that became symptomatic due to spontaneous arrhythmias (33/162, mean age 20 ± 8.6 years), SVT was documented in three-quarters (25/162) and AF in a quarter (8/162). VF was documented in 1.8 % of patients (3/162), all of whom had previously documented AF; two were aborted SCDs and one was a sudden death. The group of patients that became symptomatic were younger and had a significantly shorter AERP (246.6 ± 27.5 milliseconds) than patients who continued to be asymptomatic (283 ± 29.9 milliseconds). Inducibility (sustained AVRT or AVRT triggering AF) was found to be a better predictor of arrhythmic events than AERP. All three patients who experienced VF had multiple APs, an AERP ≤250 milliseconds and were inducible (SVT triggering AF). Only 3.5 % of non-inducible patients (4/115) became symptomatic during follow-up, suggesting that non-inducibility has a good negative predictive value.
In 2009, Santinelli et al. presented the results of a longer prospective observational study of 293 adults with asymptomatic pre-excitation (median age 36 years, 61.4 % male) in whom a baseline EP study evaluating inducibility was performed (reproducible induction of AVRT or AF lasting at least 1 minute).20 The occurrence of a first arrhythmic event was determined during a median follow-up of 67 months (range 8–90 months). Arrhythmic events appeared within a median follow-up of 27 months (range 8–55 months) in 10.5 % of patients (31/293) with a median age of 25 years: 5 % had AVRT (14/293) and 5.5 % had potentially life-threatening arrhythmias (1.5 % AVRT degenerating into AF and 4 % with AF). In the multivariate Cox analysis, younger age, AERP ≤250 milliseconds and inducibility were predictors of total and potentially life-threatening events. There was a high predictive positive value of 80 % when all three factors were present.
In the most recent, largest and longest single-centre, prospective, observational study by Pappone et al., 2,169 symptomatic and asymptomatic patients were divided into two groups according to their decision whether or not to undergo catheter ablation.44 At the end of the follow-up period (>8 years), a total of 756 patients (35 %) were asymptomatic: 206 that were ablated and 550 that were not. After a median follow-up of 22 months (range 15–41 years), 13 non-ablated asymptomatic patients developed VF (aborted cardiac arrest in all cases). An additional 48 asymptomatic patients experienced AF with a SPERRI ≤250 milliseconds after a median follow-up of 46.5 months (range 36.0–58.5 months). All of these patients were successfully ablated immediately after the event. All 13 asymptomatic patients that experienced VF had a SPERRI ≤240 milliseconds, multiple APs were present in 31 % (4/13) and 69 % (9/13) were inducible for AVRT triggering AF. Cox proportional hazards model showed that VF and AF with a SPERRI ≤250 milliseconds were independently associated with short AERP (≤240 milliseconds) and inducibility of AVRT degenerating into AF.
Di Mambro et al. showed that the EP properties of APs, including the SPERRI, assessed by trans-oesophageal EP study in asymptomatic paediatric patients (n=73) were similar to symptomatic patients (n=51).56 The only difference was a higher rate of orthodromic AVRT non-rest inducibility (mostly isoproterenol infusion) in symptomatic patients. They concluded that the potential risk of SCD in asymptomatic patients based on SPERRI measurement seems to be similar to that in symptomatic patients but that asymptomatic individuals were ‘protected’ by a lower rate of AVRT inducibility.
Isoproterenol Challenge
Observational data have shown that isoproterenol can modify the EP properties of APs and inducibility of supraventricular arrhythmia in patients with ventricular pre-excitation. Pauriah et al. demonstrated a significantly shorter atrial CL with one-to-one conduction (194 ± 15 milliseconds versus 223 ± 30 milliseconds) and AERP (191 ± 18 versus 225 ± 28) under isoproterenol in severely symptomatic adult patients while no difference in inducibility was found.57 De Ponti et al. observed a significantly shorter SPERRI and AERP in a group of 40 asymptomatic patients with persistent pre-excitation at exercise stress test who had a SPERRI >250 milliseconds and no AVRT inducible at baseline.58 Kubus et al. identified an additional 36.4 % of high-risk patients (SPERRI/shortest cycle length with one-to-one conduction ≤250 milliseconds or AERP ≤250 milliseconds; or STV inducibility) with isoproterenol when high-risk parameters were absent at baseline EP study in a group of 85 asymptomatic paediatric patients with persistent pre-excitation at maximum exercise.59 According to the recommendations for competitive sports participation in athletes with cardiovascular disease, asymptomatic athletes with pre-excitation and a SPERRI <220 milliseconds during effort or isoproterenol infusion are considered to be at increased risk of SCD.60 When conscious sedation/general anaesthesia is used, particularly in children, the use of isoproterenol may evoke real adrenergic stimulation and can better characterise the anterograde conduction of the AP. Although the role of isoproterenol challenge has yet to be clearly defined, it seems reasonable to look for high-risk EP parameters under isoproterenol when they are absent at baseline EP study.
Multiple Accessory Pathways
The recognition of multiple APs can be difficult without a complete EP study. Although in some studies this variable was not associated with higher risk,37,61 most of the data have shown that the presence of multiple APs is a risk factor for SCD.32,36,62,63
Catheter Ablation
Catheter ablation is an effective and safe method of curing arrhythmias related to APs. Data derived from single-centre experience, multicentre prospective studies and registries have shown a success rate ≥90 % and a low rate of complications (≤ 5 %).64–71 However, fatal complications related to the procedure have been communicated. Hindricks et al. reported 0.13 % mortality in a group of 222 patients: one patient died because of massive stroke 8 days after ablation, another patient developed lethal cardiac tamponade 3 days after the procedure and one patient died suddenly 24 days after ablation.66 Scheinman et al. reported four deaths following a total of 5,427 AP ablations (0.08 %).67 Lu et al. reported 0.16 % mortality associated with WPW ablation (4/2,527 in an 11-year period).11 More recently, one death due to embolism in a patient with Ebstein’s anomaly was reported in 4,603 AP ablations (0.08 %) by the First Latin American Catheter Ablation Registry.71
The result of prophylactic catheter ablation in asymptomatic patients was first evaluated in a randomised clinical trial of patients from two centres in 2003.26 A total of 76 high-risk patients (≤35 years old with reproducibly-induced arrhythmias) out of 224 eligible patients were enrolled. In the final analysis, 35 control patients and 37 ablation patients were compared. In a median of 27 months (range 9–60 months) of follow-up, there were two arrhythmic events (5 %) in the ablation group where the atrioventricular nodal re-entrant tachycardia was successfully ablated. After a median follow-up of 15 months (range 8–53 months), 60 % of the control group patients had experienced an arrhythmic event (15 had SVT, five had AF and one had VF as a first manifestation with previous AVRT triggering AF, a SPERRI of 200 milliseconds and multiple septal APs). The estimated 5-year arrhythmic event rate was significantly higher in the control group (77 %) than the ablation group (7 %); ablation led to a risk reduction of 92 %. No major complications were observed in the ablation group and no patients died.
In 2004, a randomised clinical trial evaluated the results of prophylactic catheter ablation in children with asymptomatic pre-excitation.63 Out of 165 eligible patients aged 5–12 years, 60 high-risk patients (with reproducibly-induced ARVT or AF) were randomised to control or ablation. During a median follow-up of 34 months (range 19–44 months), 27 control patients and 20 ablation patients were compared. One 11-year-old ablation patient had an atrioventricular reciprocant tachycardia (AVRT) 21 months after the ablation of two APs. In contrast, after a median follow-up of 19 months (range 16–22 months), an arrhythmic event was detected in 44 % of the control patients (12/27). Of these, symptomatic arrhythmias (AVRT in five patients and AF in two patients) were documented in 26 % of patients (six boys and one girl) and Holter-documented silent episodes of sustained AF were observed in 18 % (five patients). After declining radiofrequency ablation, one 10-year-old died suddenly and another two patients experienced VF. All three patients were boys, had multiple APs and inducible AVRT and AF. The freedom from arrhythmic events was significantly higher in the ablation group compared with the control group. Independent predictors of arrhythmic events were the absence of prophylactic ablation (hazard ratio [HR] 69.4; 95 % confidence interval [CI] 5.1–950; p=0.001) and multiple accessory pathways (HR 12.1; 95 % CI 1.7–88.2; p=0.01). The estimated number of high-risk patients needed to treat to prevent arrhythmic events in one patient was two (95 % CI 1.4 to 3.1). Although there was a high percentage of total complications associated with EP study (15 %) and catheter ablation (5 %), most were minor complications and no death occurred.
In the largest and longest single-centre, prospective, observational study, Pappone et al. observed a success rate of 98.5 % in the ablation group (among the 1,168 patients, 206 were asymptomatic), no deaths were associated with the procedure and there was a very low rate of major complications (complete atrioventricular block in 0.08 %). Kaplan–Meier analysis at 8 years showed that catheter ablation was associated with a significantly better survival free from malignant arrhythmias (AF lasting >1 minute with a SPERRI ≤250 milliseconds) and from VF.
Discussion
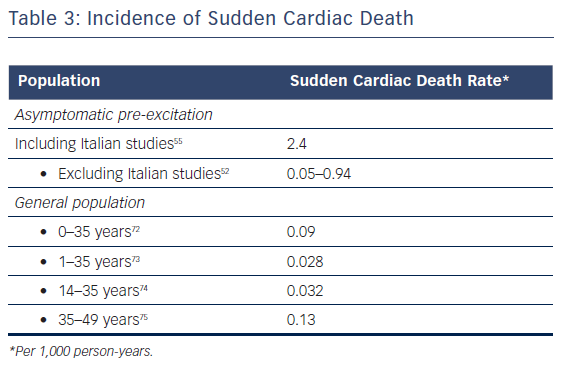
There are varying epidemiological data on the risk of SCD in the natural history of asymptomatic patients. When some populations are excluded (i.e. Italian studies), the rate of SCD is similar to that observed in the general population. Therefore, it is possible that the real incidence of SCD is still unknown, see Table 3.44,72-75 Although low or very low, the presence of pre-excitation in an asymptomatic, generally young, healthy people adds a definite risk of SCD. There is also a very low but definite risk of mortality associated with catheter ablation. Although evidence from randomised clinical trials demonstrates the benefit of catheter ablation, it may not support a Class I indication because these studies are not multicentre trials and the number of patients included is low.26,63 Consequently, a large, long-term, multicentre randomised clinical trial of catheter ablation in asymptomatic patients would provide the best evidence on which to recommend treatment. However, for many reasons, this kind of study maybe never be performed.
Chevalier et al. performed a sensitivity analysis to compare two management strategies in asymptomatic patients: no treatment and catheter ablation. The hypothetical study – which was scheduled to run for 10 years – was based on a decision tree model, the hypothetical population – aged between 20 and 40 years – did not undergo an initial risk stratification, and the risk of recurrence after initially successful catheter ablation and cryotherapy were not taken into consideration.76 They concluded that when the risk of sudden death was at least 1.5 out of 1,000 patients per year, radiofrequency catheter ablation with a success rate of 95 % is superior to abstention. However, the result of this study has not been widely accepted.76
Limitations in current evidence have led to continued debate about what to recommend these patients. Current guidelines recommend an EP study as a Class IIA (level of evidence B/C) indication when loss of pre-excitation is not observed during non-invasive risk stratification. Catheter ablation is a Class IIA (level of evidence B/C) indication for young asymptomatic patients (aged 8–21 years) with WPW pattern when a SPERRI is ≤250 ms during induced AF at an EP study, whatever the risk of the procedure when localisation of the APs has been taken into account.77 The most recent American College of Cardiology/American Heart Foundation/Heart Rhythm Society guideline recommends catheter ablation as a Class IIA recommendation in asymptomatic patients when an EP study (also a Class IIA recommendation) stratifies the patient at high-risk for arrhythmic events or if the pre-excitation precludes specific employment (such as pilots). Although the level of evidence supporting the indication is considered B-NR (moderate-quality evidence from nonrandomised, observational or registry studies), one of the studies included is the only randomised clinical trial demonstrating the benefit of prophylactic catheter ablation in patients of ≥13 years of age. A recent systematic review, including nine previously analysed studies (eight uncontrolled prospective cohort studies), concluded that an EP study for risk stratification along with AP ablation in cases with a high risk of future arrhythmias may be beneficial.78
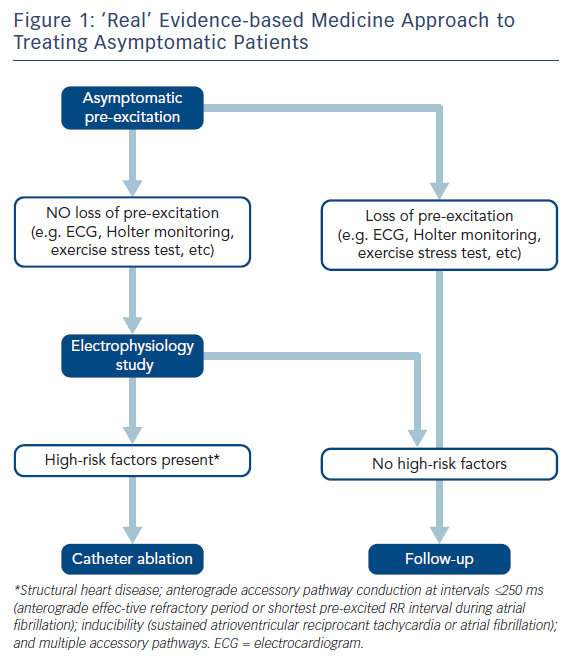
The main argument against studying and treating asymptomatic patients has been the poor predictive accuracy (low specificity and low positive predictive value) of non-invasive and invasive risk stratifiers due to the low event rate of SCD. Many patients would be unnecessarily treated and exposed to the risks of EP study and catheter ablation if all such asymptomatic patients were treated. A negative predictive value of <100 % will leave some patients exposed to an avoidable risk of SCD and according to published surveys, in clinical practice most electrophysiologists choose to invasively stratify and ablate this group of asymptomatic patients.79,80 Pappone et al. showed that 70 % of electrophysiologists performed an EP study for risk stratification and prophylactic ablation80 and Campbell et al. that 84 % of paediatric electrophysiologists used some form of EP study to risk-stratify asymptomatic children with ventricular pre-excitation.79 Consequently, and taking into account these data, most electrophysiologists are managing asymptomatic patients according to ‘real’ evidence-based medicine.2 After a critical analysis of the available evidence, expert judgment, discussing the risks and benefits of the procedure – including potential benefits beyond mortality, such as better quality of life – an EP study to stratify risk and an ablation procedure should be considered when accepted high-risk factors are present and whenever the risk of complications judged by localisation is low (i.e. atrioventricular block risk in the para-Hisian and mid-septal regions), see Figure 1. This strategy seems to be more accepted than conservative management in clinical practice.