Ischaemic stroke remains a leading cause of mortality and morbidity globally. The association between AF and stroke has long been established. Each year, AF-related cardio-embolic stroke accounts for up to one-quarter of ischaemic strokes in the US.1
The mechanisms behind cardio-embolic stroke in AF are not completely understood and are thought to involve blood stasis, hypercoagulability and tissue injury, similar to what is seen in other thrombotic phenomena in vascular beds, as previously described by Virchow.2
Atrial fibrosis plays a central role in creating a substrate for AF.3 Fibrosis results from myocyte loss and replacement with collagen in the extracellular space. This leads to increased heterogeneity in the atrial tissue which, coupled with atrial myocyte electrical remodelling, alters the anisotropy and conduction velocity of electrical wave propagation within the atrial myocardium, resulting in an increased probability of arrhythmia initiation and sustenance, in line with the concept of atrial myopathy presented by Goette et al.4,5
The relationship between atrial fibrosis and stroke is garnering more attention in view of supporting evidence from the AF patient population. New research in patients with embolic stroke of undetermined source (ESUS) also suggests a relationship between atrial fibrosis and stroke independent of the presence of AF.
In this review, we discuss the available evidence linking atrial fibrosis to stroke in patients with and without known AF, as well as new evidence linking atrial fibrosis with new-onset AF and recurrent stroke in patients with ESUS. The evidence presented forms the foundation of a new paradigm in the relationship between atrial arrhythmia and stroke, where fibrosis is central to this relationship and is at the nexus between these two clinically important problems.
AF and Embolic Stroke: is AF necessary?
The classical mechanistic relationship between AF and stroke stipulates that AF, in the presence of cardiovascular risk factors, leads to stasis and hypercoagulability with subsequent thrombus formation and systemic embolisation. Patients with persistent and permanent patterns of AF, typically associated with a higher burden of arrhythmia, have a higher risk of stroke than patients with paroxysmal forms.6 A higher burden of AF has also been associated with a higher stroke risk in patients with paroxysmal AF.6
Suppression of AF with catheter ablation has been associated with a reduction in stroke risk in observational studies, and in a recent randomised trial of persistent and long-standing AF.7,8 Early rhythm control of patients within a year of diagnosis with AF has also been associated with stroke reduction.9
These findings support a causal association between AF and stroke. This is in contrast to other studies of patients with ICDs, such as pacemakers and implantable defibrillators, which have argued against causality because of the observed temporal dissociation of AF episodes with thrombo-embolic events.10
Pacemakers and ICDs with atrial leads allowing for continuous atrial arrhythmia monitoring provide a high-fidelity rhythm recording of atrial arrhythmias including AF, and are a unique source of information for studying the temporal relationship between atrial arrhythmias and thromboembolic events, including stroke.
Several studies examining this temporal association have shown that there were no device-detected atrial arrhythmia episodes preceding thrombo-embolic events in a large majority of patients.11,12 In another study, oral anticoagulation with vitamin K antagonists was started and discontinued as device-detected episodes of arrhythmia failed to show benefits of this approach and also demonstrated a temporal dissociation between AF episodes and thromboembolic events.13 These findings suggest that, in this population of patients with ICDs, the presence of AF is not an essential component of the mechanism of thromboembolic events.
Against this background, an alternative explanation has been proposed. This is based on the presence of underlying atrial disease manifesting with symptomatic bradycardia, hence requiring a ICD and, simultaneously, predisposing these patients to thrombo-emboli independent from atrial arrhythmic events.
Another stroke population where the association between atrial disease and stroke has been examined is ESUS, which is a subset of cryptogenic stroke. AF has long been suspected in these patients as a potential cause of cardiac thrombo-emboli. Long-term cardiac monitoring in patients with cryptogenic stroke has revealed that longer monitoring is associated with higher detection rates of AF.14 However, even using a relatively short duration of episodes (30 seconds), the rate of incident AF was about 30% after three years of monitoring using an implantable loop recorder in the CRYSTAL AF study.15 Another way of stating these findings is that 70% of these cryptogenic stroke patients did not have detectable arrhythmia.15
These findings also call into question the role of AF in thrombo-embolic stroke in this population. In the LOOP study, anticoagulation based on detected arrhythmias using implantable loop recorders in patients with stroke risk factors did not demonstrate a beneficial decrease in stroke rates, leading the investigators to conclude that not all AF is worth screening and anticoagulating for.16
Moreover, the role of clinical risk factors in modulating stroke risk in AF and ESUS populations is not clearly understood; research has demonstrated that the CHA2DS2-VASc aggregate score is more predictive of thrombo-embolic events in the absence than the presence of AF.17
An alternative mechanism has been gaining support with new evidence from various studies. In this new model, atrial fibrosis and associated atrial disease is the nexus between arrhythmia and thrombo-embolic phenomena. This new paradigm is supported by evidence of biomarker activation, atrial enlargement, contractile dysfunction and increased atrial fibrosis in patients with stroke, including ESUS, as well as AF.18,19 We discuss the available evidence in the paragraphs below.
Biomarkers of Fibrosis and Association with Stroke
Many biomarkers involved in the coagulation cascade and subsequent thrombolysis, including thrombin, fibrin and D-dimer, have been associated with cardioembolic stroke.20,21 Other biomarkers of cardiac dysfunction – including abnormal extracellular matrix homeostasis, which is linked to abnormal collagen turnover and subsequent fibrosis – have also been proposed.
Marìn et al. have demonstrated abnormal ratios of matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with AF, but a direct link to stroke is lacking.22 Similarly, pro-collagen type III N-terminal pro-peptide (PIIINP), C-telopeptide of type I collagen (ICTP), fibroblast growth factor 23 (FGF-23) and galactin-3 (gal-3) have been studied as biomarkers of fibrosis in patients with AF and linked to arrhythmia recurrence after catheter ablation, but have not been studied in stroke in the setting of AF or other ischaemic strokes.23–25
Cardiac troponin and N-terminal-pro-B-type natriuretic peptide (BNP) have been linked to cardioembolic stroke, and ESUS in large cohort studies, including the MESA and the ARIC studies.26–29 BNP is a neurohormone that is primarily released from the ventricles in response to increased wall tension and stretch. It has diuretic (natriuretic) properties and attenuates the renin–angiotensin–aldosterone response, resulting in smooth muscle relaxation in blood vessels. It is synthesised as a pro-hormone (pro-BNP) and is later cleaved into a C-terminal and an N-terminal (NT)-proBNP fragments. Identifying elevated levels of NT-pro-BNP can be useful in the setting of ischaemic stroke, especially cryptogenic stroke, where they may indicate the presence of heart failure and AF, both important risk factors for cardiac embolism.30 It is important to note that, while these biomarkers suggest myocyte loss and subsequent atrial fibrosis, a direct link to thrombus formation is still lacking.
ECG Evidence of Fibrosis and Relationship with Stroke
The 12-lead ECG is a widely available tool that is commonly used in the setting of stroke to evaluate patients for the presence of AF. In patients in sinus rhythm, the ECG can be used to evaluate for evidence of left atrial remodelling. Of multiple P wave indices, the terminal portion in lead V1 has been studied in patients with ischaemic stroke and shown to correlate with this outcome.31
The P wave terminal force in V1 (PTFV1) is the product of P wave width and depth and can be calculated either manually or automatically on commercially available ECG signal processing software. Abnormal PTFV1 has been shown to be associated with incident ischaemic stroke in a number of studies.32 While PTFV1 appears to be a useful ECG marker for LA remodelling, its specific relationship with atrial fibrosis has not been studied. Moreover, the temporal stability of this index is not well studied.
Echocardiographic Assessment of Atrial Size and Function
Basic echocardiographic assessment of atrial structure includes atrial diameters and surface area in various views. Larger anterior–posterior atrial diameter in the parasternal long axis view and the left atrial area in the four-chamber view have been associated with stroke risk with or without AF.33,34 Similarly, larger atrial volume indexed to the body surface area has also been associated with ischaemic stroke.
More advanced echocardiographic measures, such as speckle tracking, can detect more subtle abnormalities in atrial function. Atrial reservoir dysfunction, a sign of reduced LA compliance, assessed using this approach has been associated with cryptogenic stroke.35 These atrial mechanical and contractile abnormalities measured using atrial strain and strain rate on echocardiography have also been associated with atrial fibrosis quantified using cardiac MRI.36
Cardiac MRI Assessment of Atrial Shape and Contractile Function
Feature tracking using cardiac MRI has been used to examine atrial relaxation and contraction. Atrial reservoir function and atrial emptying fraction were found to be associated with ischaemic stroke in the absence of prevalent or incident AF.37
Moreover, atrial shape deformation with increased atrial sphericity leading to abnormal blood-flow patterns in the left atrium and left atrial appendage have been proposed to have a role in thrombogenesis.38 These findings further support the concept of atrial disease or atrial myopathy as a contributor along the causative pathway of ischaemic stroke independent of AF.39
Cardiac MRI Fibrosis Quantification
MRI for the assessment of cardiac fibrosis is commonly used in the work-up and assessment of various types of cardiomyopathies. Late-gadolinium enhancement MRI (LGE-MRI) is an established technique that leverages the washout kinetics of gadolinium, an extracellular contrast agent. The correlation of LGE-MRI-identified fibrosis with ventricular myocardial pathology has been established.40
Applying this imaging technique to the left atrium is more difficult owing to the thin atrial wall and the special resolution of this imaging modality. Our previous research has correlated LGE-MRI fibrosis of the left atrium with an abundance of collagen on atrial biopsies and demonstrated a strong correlation with catheter ablation outcomes in AF.41,42
Fibrosis detected by LGE-MRI has been demonstrated to be significantly higher in patients with a prior history of stroke, as well as with new-onset major adverse cardiovascular events including ischaemic stroke.43,44 Atrial fibrosis was also found to be elevated in patients with left atrial appendage thrombi found on transoesophageal echocardiographic evaluation.45
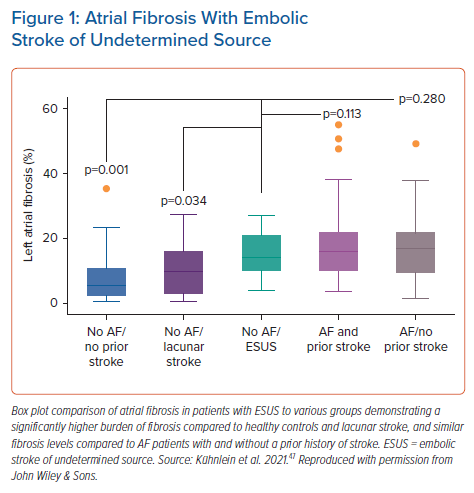
In patients without known AF, atrial fibrosis has been demonstrated to be more pronounced in ESUS patients than in age- and sex-matched controls to a degree similar to that seen in AF, suggesting that atrial fibrosis may be in the causative pathway linking AF and stroke.46 In a larger study, atrial fibrosis was found to be significantly higher in patients with ESUS than in lacunar stroke patients and control subjects and comparable to that seen in patients with known AF (Figure 1).47
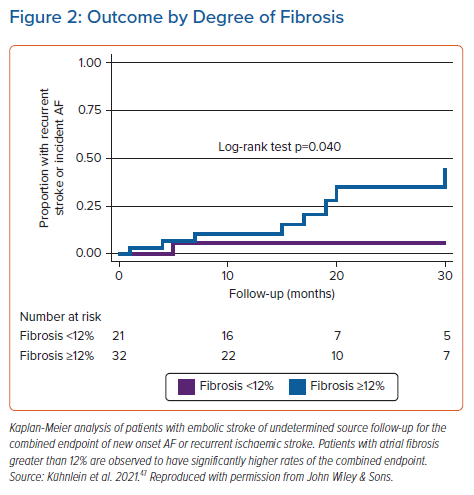
In a prospective study, patients with ESUS underwent LGE-MRI quantification of left atrial fibrosis and were followed prospectively for recurrent stroke or incident AF.47 Those with atrial fibrosis ≥12% were found to have a 4.3-fold increase in the risk of the combined outcome of recurrent stroke or new-onset AF compared to those with fibrosis <12%, after an average follow-up period of 19 months (Figure 2). The potential implications of this study may be significant in patients with ESUS, as atrial fibrosis could be a patient selection tool for oral anticoagulation for secondary prevention. In addition, atrial fibrosis may be a therapeutic target to reduce the risk of stroke and new-onset AF.
The NAVIGATE-ESUS and RESPECT-ESUS clinical trials randomized patients with ESUS to aspirin or oral anticoagulation therapy with rivaroxaban and dabigatran, respectively.48,49 Both studies showed no significant benefit in oral anticoagulation when using no atrial disease-specific selection strategy. In a post-hoc analysis of the NAVIGATE-ESUS trial, patients with an increased atrial surface area were found to benefit from OAC, indicating that people with atrial disease may benefit from such therapy.50
Selecting patients based on suspected atrial disease for anticoagulation is being studied in the ARCADIA clinical trial, where patients with evidence of left atrial enlargement on echocardiography, PTFV1 or elevated plasma NT-pro-BNP will be randomised to receive either aspirin or oral anticoagulation with apixaban.51 Other trials using elevated LGE-MRI detected atrial fibrosis for selection for oral anticoagulation are also in preparation and will be launched soon.
Conclusion
Atrial fibrosis is a multifactorial phenomenon with measurable abnormalities in biomarkers and cardiac imaging. While current evidence suggests there are links between atrial fibrosis and both atrial arrhythmia and thromboembolic disease, the mechanism by which fibrosis leads to thrombus formation and predisposition to embolisation including stroke is still not completely understood. It is also plausible, since fibrosis is associated with many cardiovascular risk factors, that it may be a marker of overall cardiovascular risk including stroke.
In the presence of AF, fibrosis may play an important role in creating a state of electromechanical dysfunction that leads to stasis and localised contractile dysfunction that may form a nidus for thrombus formation.52 Fibrosis may also be implicated in the pathogenesis of a hypercoagulable state.21 In the absence of AF, electromechanical dysfunction and altered blood flow dynamics may also be at play. Research to understand these mechanisms is under way using computational modelling of arrhythmia and blood-flow dynamics in tissue fibrosis obtained through cardiac imaging.51
Clinical Perspective
- Embolic stroke of undetermined source (ESUS) is a clinical challenge for neurologists and cardiologists as the rate of AF detection is low and the risk of recurrent stroke is high.
- Evidence suggests a link between atrial fibrosis and ESUS independent of AF, which suggests that fibrosis is on the causal pathway for thrombo-embolisation.
- Atrial fibrosis-based strategies for risk stratification and secondary prevention of ischaemic stroke are needed.