There is a large proportion of the population who are affected by syncope, and this underscores the importance of identifying patients with syncope who are at higher risk of adverse outcomes, both for their safety and the optimal use of healthcare resources. Many algorithms have been proposed for the risk stratification of syncopal patients and for identifying patients who would benefit from a more extensive diagnostic work-up.1 In the absence of structural heart disease or cardiac rhythm disturbances, neurally mediated syncope (NMS) appears to be the most likely diagnosis. However, in one-third of cases, even after a detailed evaluation, the aetiology and mechanisms of syncope remain elusive and it is characterised as syncope of unknown origin (SUO).2 The adenosine (AD) test (ADT) has been proposed as a useful tool in investigating the pathogenesis of SUO. By exhibiting different responses to ADT, distinct subgroups may show a differential involvement of AD and its receptors in the pathophysiology of syncope helping to decide group-targeted therapeutic strategies.1,3
This article aims to present the evidence for the use of ADT to investigate unexplained syncope and to find out whether the outcome of the test could influence therapeutic strategies. We also present existing evidence on AD plasma (ADP) levels and its receptors due to the involvement of the adenosine pathway in the ADT response. A literature search was performed to identify relevant reviews and studies in this field. We used PubMed and searched for papers including the terms ADT, SUO, NMS, AD and AD receptors. We reviewed relevant reports and their reference lists, ranging from 1990 to 2021.
The Role of Adenosine in the Pathophysiology of Syncope
AD is a purine nucleoside molecule that consists of adenine and ribose. Its endogenous precursor is a nucleoside triphosphate called adenosine triphosphate (ATP). AD acts intracellularly and extracellularly and, together with its receptors, mediates a variety of adaptive responses in the cardiovascular system in normal or pathological conditions. AD seems to increase in cases of exaggerated metabolic demand in local tissue and, more specifically, in cases of low blood flow or hypoxia.4,5
AD and ATP exert their signalling effects by binding to two purine receptors on the cell membrane, called adenosine P1 receptors and ATP or P2 receptors. P1 receptors are G-protein receptors and are further classified into A1R, A2AR, A2BR and A3R. Two subtypes of P2 receptors have been identified: P2X, which are ion channels, and P2Y which bind to G proteins. This interaction between AD or ATP and their receptors, makes up the so-called purinergic signalling, usually found in the pathogenetic mechanism of syncope.6–8
In the conduction system, ATP and AD exert a negative chronotropic and dromotropic effect, suppressing sinus node automaticity and prolonging conduction through the atrioventricular (AV) node.5 Also, AD causes a sympathetic withdrawal that may result in syncope in susceptible individuals. Two types of receptors seem to play a crucial role in syncope, the high-affinity A1R and the low-affinity A2R. A1R are mostly located in the sinoatrial node and the AV node and are mainly responsible for bradycardia-induced syncope. A2R – and more specifically A2AR – are located in high densities in vessels and are responsible for vasodilation during syncope.3,5
Several studies have investigated the role of AD as a modulator of syncope via A1R and A2R and how ADP levels and adenosine receptors’ expression might affect the outcome of the head-up tilt table test (HUTT) and ADT.3,5 Endogenous AD is comparable to isoproterenol – the synthetic compound used in the tilt-table test – and can trigger a vasovagal response during HUTT in susceptible patients.9 Deharo et al. supported the hypothesis that different purinergic profiles could differentiate HUTT and ADT outcomes.10,11 As for ADP levels, they were higher in patients with positive HUTT and lower in cases of positive ADT. The lowest levels were observed in positive ADT and negative HUTT and highest in cases of negative ADT and positive HUTT. Both positive or both negative tests related to intermediate ADP levels.10,11
Like many other cell surface receptors, the number of AD receptors undergoes up- or down-regulation according to the duration of exposure to low or raised concentrations of AD, respectively. For example, an increased expression and up-regulation of A2AR has been noted in patients with a positive HUTT.12 Such an adjustment seems to play a specific and critical role in the pathogenesis and manifestation of syncope – and in the outcomes of ADT.3–5,8
In light of previous studies, ADP and AD receptor levels have been used to identify patients with syncope without structural heart disease in two distinct subgroups (Table 1):
- Low-adenosine syncope: syncopal patients with low baseline ADP and a low expression of A2AR. These patients usually exhibit a negative HUTT and a positive ADT although an overlap between the two tests has also been reported. Low ADP makes these patients susceptible to a sudden increase of spontaneously released AD, leading to syncope without prodromes due to a complete AV block or sinus arrest. A stimulation of the high-affinity A1R, which are free in cases of low ADP, have been predominantly implicated.3,10,11
- High-adenosine syncope: patients with high baseline ADP and a high expression of A2AR. These patients exhibit a positive HUTT and a negative ADT. Clinically, they manifest NMS with typical prodromes. The high levels of spontaneously released AD are unable to affect the already saturated A1R. However, the effect of AD on A2AR causes vasodilation as a result of vagal stimulation and sympathetic inhibition leading eventually to syncope.3,10,11,13
Methodology and Interpretation of Adenosine Test
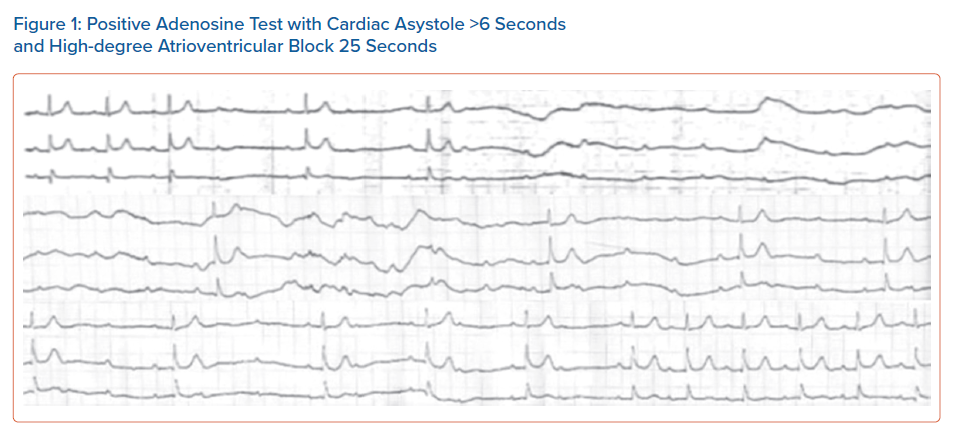
The test is performed with the patient in a supine position under continuous ECG monitoring. A bolus dose of 0.15 mg/kg AD in a large antecubital or femoral vein is followed by rapid administration of 20 ml saline flush. Induction of cardiac asystole of ≥6 seconds or a high degree AV block of ≥ 10 seconds, is considered abnormal and the test is characterised as positive (Figure 1).5,11,13
ADT seems to be quite safe because any side-effects, such as facial flushing, headache or chest discomfort, are transient, lasting for up to one minute. Asthmatic patients deserve special consideration to avoid exacerbating the condition. AF can be occasionally induced, while an extremely rare, bradycardia-induced polymorphic tachycardia, mostly in patients with long QT, has been reported as a dangerous proarrhythmia that can occur after ATP or AD administration.14,15
The Current Status of the Adenosine Test
ADT has never gained wide clinical acceptance because it has been challenged by studies that doubted the clinical significance of asystole or a high-degree AV block occurring during the test, as being the predominant mechanism responsible for spontaneous syncope.2 Some of these studies that used an implantable loop recorder (ILR) in syncopal patients did not confirm the expected paroxysmal AV block at the time of syncope, even in cases of a previous positive ADT with long pauses.2,16–18 Moreover, the incidence of syncopal recurrences did not show any difference between patients with positive and negative ADT.16 Even in paced syncopal patients, no correlation of syncope recurrences with ADT positivity was noted.19
According to the above findings, it was concluded that ADT does not provide predictive value for patients with SUO or when selecting patients that would benefit from pacemaker implantation.2,16,19 Following the above evidence, the recommendation to use ADT as a routine investigation for SUO was removed from the last European Society of Cardiology (ESC) guidelines for the investigation and management of syncope.13
Adenosine Test as a Complementary Test to Head-up Tilt Table Test in Syncope Diagnostic Work-up
HUTT is frequently used as part of the diagnostic algorithm for patients with syncope. According to the latest European Society of Cardiology guidelines for syncope, it possesses a class IIa indication for diagnostic purposes.13 The test should be considered:
- to confirm the diagnosis of NMS in patients in whom the diagnosis has been suspected but not confirmed by initial evaluation; and
- for the assessment of autonomic failure, delayed orthostatic hypotension and postural orthostatic tachycardia syndrome.20
HUTT could also be helpful in discriminating syncope from psychogenic syndromes or epileptic seizures while it might also have an educational role and be used to reassure the patient.13,20 According to recent guidelines, HUTT is not necessary when the clinical presentation of syncope is typical of NMS, implying that its major diagnostic value is for those cases categorised as SUO.13 However, HUTT is of limited value in syncope without prodromes as well as in atypical syncope with uncertain or absent triggers. This is because no reproduction of spontaneous syncope can be accomplished in patients with a history of syncope without prodromes since the patient cannot recall the event.
Also, a limitation of HUTT is that the provoked syncope could be entirely different from the clinical one. Therefore, additional diagnostic testing that could provide further information about the syncope mechanism is warranted.11,13,20
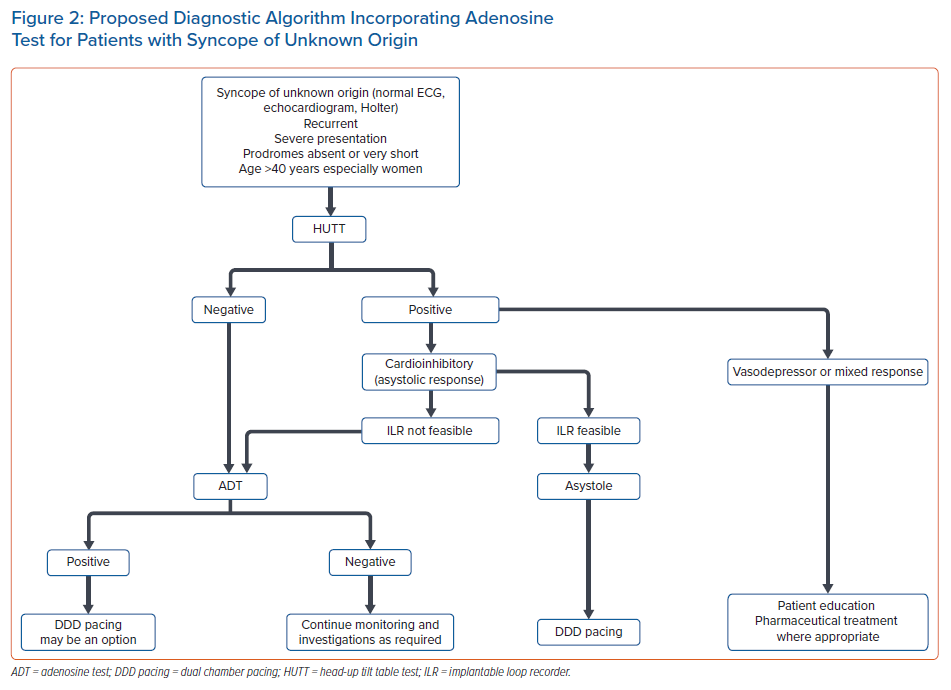
ADT could have an additional role to HUTT in the diagnostic work-up of syncope. Although data from studies have failed to prove a consistent correlation between ADT and the rhythm disturbances occurring during spontaneous syncope, ADT can shed further light on the purinergic profile of syncopal patients and the results can have therapeutic implications.2,3
ADT could be used to complement HUTT in the following cases:
- Women over 40 years with a normal heart and a short history of SUO without prodrome symptoms and sudden spells when upright, usually leading to injury. A positive ADT in this group of patients – especially in cases where there has been a negative HUTT – may reveal an adenosine sensitivity, which has implications for syncope management in this group, suggesting, for example, DDD pacing instead of conservative therapy.21–23
- HUTT-positive patients with a strong cardio-inhibitory response. This mostly applies to patients over 40 years where pacemaker implantation could be an option. Although the best strategy in these patients is ILR implantation to confirm asystole during syncope, ADT could also play a supplementary role, especially in cases where ILR implantation is not a feasible option. A positive ADT could predict probable long pauses during spontaneous syncope, therefore supporting a decision on pacemaker implantation.16,24
Taken together, the above pieces of evidence may be interpreted as follows:
- In cases with negative HUTT and negative ADT, the aetiology of syncope remains undetermined and monitoring and investigations should continue as required, while the diagnosis of reflex syncope cannot be excluded.
- In cases of negative HUTT and positive ADT, especially in women >40 years without prodromes, efforts should be made to document paroxysmal AV block which may then justify permanent pacing or, depending on AD levels, administration of xanthine derivatives.
- In cases of positive HUTT advocating reflex syncope with a concomitant negative ADT, treatment should be tailored according to the severity and rate of recurrence of the episodes and also depending on the clinical phenotype (low blood pressure, prodromal symptoms, dominant cardioinhibition) and age, as per the ESC guidelines.13
- In the subgroup of cases of positive HUTT and positive ADT, especially in cases of cardioinhibitory HUTT, an ILR implantation should be sought, but permanent pacing might be considered where this is not feasible.
Therapy Guided by the Adenosine Test
A major challenge regarding the ADT is the interpretation of a positive test result. It remains unclear whether a positive ADT indicates an underlying clinically significant cardioinhibitory NMS or unveils a mostly dormant conduction system disease that may spontaneously present with syncopal or presyncopal attacks.19 Some investigators support the concept of ADT unmasking idiopathic paroxysmal AV block, while other groups suggest that ADT can identify bradycardia-related pacing indications, such as sinus node disease and conduction system disease.11,21,25,26
There are also pilot studies supporting the role of ADT to identify those patients with NMS who may benefit from pacing therapy.27 According to the ISSUE-3 study, DDD pacing is effective in cardioinhibitory syncope with ILR-documented asystole during spontaneous syncope.24 This subgroup of patients – >40 years, mostly women, experiencing syncope without prodromes or with very short-lived prodromes, normal heart and normal ECG – may be more frequently characterised by a low-adenosine profile and a positive ADT.2,3,5,21 Therefore, ADT may possess a surrogate role in identifying this specific group of patients with asystolic syncope, especially when an ILR implantation is not feasible, and serve as a supplementary, easily performed and low-cost diagnostic test that can facilitate clinical decision-making. A proposed diagnostic algorithm incorporating ADT for patients with syncope of unknown origin is presented in Figure 2.
In patients with a low-adenosine profile of syncope who are quite susceptible to exogenous AD, few studies indicate that offering adenosine receptor antagonists, such as xanthine derivatives, would help prevent syncopal recurrences.28–30 Theophylline appeared quite promising in adenosine-mediated syncope, more specifically in preventing idiopathic AV block, delaying pacemaker implantation, or acting as a bridging therapy to pacemaker implantation, especially in patients <40 years old who do not seem to benefit from a permanent pacemaker.3,11,20 It should be noted however that in the above studies, therapy with theophylline was guided by AD levels rather than by the results of ADT. Conversely, syncopal patients with typical NMS and negative ADT, do not seem to benefit from adenosine receptor antagonists or pacemaker implantation and other strategies are proposed that mainly centre on patient education. Pharmaceutical therapies such as fludrocortisone or midodrine would be of partial benefit, along with autonomic educational skills, such as tilt training (Table 1).3,13,20,23,31
Conclusion
ADT has a role in the diagnostic approach to SUO. Its correlation with ADP levels and A2AR could reveal particular subgroups of patients with syncope with different characteristics that would benefit from different therapeutic strategies. This can contribute to a more personalised approach to patients with syncope and allow the introduction of targeted therapies. Undoubtedly, more research and further studies in large populations might shed light on neuro-hormonal and purinergic signalling in NMS and SUO and may endorse the ADT test along with the measurement of AD and its receptors as guiding tools for more accurate diagnosis and treatment of syncope.
Clinical Perspective
- Syncope is a complex clinical condition.
- The adenosine test (ADT) and the head-up tilt table test could be used together for the diagnosis of syncope of unknown origin (SUO).
- Adenosine plasma (ADP) levels and adenosine receptors may reveal distinct subgroups of syncopal patients.
- ADT, ADP levels and adenosine receptors could serve in the investigation and management of SUO.