Supraventricular tachycardias (SVTs) other than AF are common in the general population at an estimated incidence of 73 per 100,000 and prevalence of 140 per 100,000 people.1 Meanwhile, AF is the most common cardiac arrhythmia with a lifetime risk of around one in four in the Western world.2 Ventricular tachycardia (VT) is a life-threatening cardiac arrhythmia and a major cause of sudden cardiac death, the incidence of which is estimated to range from 50 to 100 per 100,000 in the general population.3
ICDs are the mainstay of therapy for ventricular tachyarrhythmias (e.g. VT or VF) and the prevention of sudden cardiac death. However, when shocks are delivered inappropriately (i.e. for rhythm disturbances other than VT or VF) they can be associated with significant morbidity, psychological distress and worse all-cause mortality.4 Although ICD shocks may increase heart failure-related hospitalisations and potentially cause direct myocardial damage, it remains unclear whether the presence of non-lethal SVTs or VTs, which themselves may be related to worsening cardiovascular morbidity and mortality, might explain this association, in part.5 Based on historical data, over a 5-year period, up to 18% of ICD recipients may receive an inappropriate shock while 80% of inappropriate shocks are caused by SVTs such as atrial flutter, sinus tachycardia, atrial tachycardia, atrioventricular re-entrant tachycardia (AVRT), AV nodal re-entrant tachycardia (AVNRT) and AF.6 With contemporary programming of ICDs with delayed high-rate detection and the use of SVT discriminators, the rates of inappropriate shock and therapy can be reduced to around 3–4%.7
Robust discrimination of SVT from VT or VF is therefore critical for optimal functionality of an ICD. This is achieved through the use of various detection algorithms developed by device manufacturers that aim to balance sensitivity (detection of potentially life-threatening VT/VF) with specificity (avoidance of inappropriate therapy). There are also benefits in utilising the diagnostic capabilities offered by the presence of atrial intracardiac electrograms (EGMs) within implanted devices. Device-detected sub-clinical AF or atrial high-rate episodes (AHREs) are characterised by one or more runs of rapid atrial arrhythmia detected by a device without the presence of symptoms or clinical AF detected on routine monitoring with 12-lead ECG or ambulatory monitoring. The presence of AHREs has been associated with worsening heart failure, thromboembolism and more cardiovascular-related hospitalisations.8 There are studies currently underway to evaluate whether the initiation of oral anticoagulation in patients with AHRE can reduce the risk of stroke or cardiovascular death.9,10 In this article, we review the detection algorithms used by the major device manufacturers to classify cardiac rhythm and the discriminators that are used to help reduce inappropriate therapies.
Sensing, Therapy Zones and Detection Windows
The correct classification of cardiac rhythm and application of discrimination algorithms relies on accurate sensing and detection. Sensing refers to the determination of the timing of cardiac depolarisation signals using filters, amplitude thresholds and blanking periods. Detection refers to the interpretation of that signal by the device, which involves classification of a sequence of sensed signals to determine the nature of the cardiac rhythm.11 Under-sensing occurs when there is a failure to detect a depolarisation, while over-sensing occurs when signals that are not part of local myocardial depolarisation are classified as such. Blanking periods can help to prevent over-sensing (e.g. a post-ventricular atrial blanking period [PVAB] that could prevent incorrect classification of AF or atrial tachycardia). Under-sensing, particularly of ventricular depolarisation, may be important in VF, in which the EGM amplitude may be below the sensing threshold, but this is rare in practice.6
Modern algorithms for delivery of ICD therapy involve a faster rate threshold and a longer duration of monitoring prior to therapy delivery, in order to prevent inappropriate or unnecessary shocks and allow some arrhythmia episodes to self-terminate.12 Tiered therapy ICD systems may have up to four VT detection zones that allow for zone-specific duration for detection and therapies. The slower zones (e.g. monitor-only zone) are classified as VT zones, while the fastest zone is conventionally classified as the VF zone. Therapies may be delivered in the VT zone if a programmed threshold is met. Additional algorithms such as a confirmation or re-detection algorithm may enable inhibition of further therapy if the episode terminates spontaneously or through application of anti-tachycardia pacing (ATP) and/or shocks.
Single-chamber Discriminators
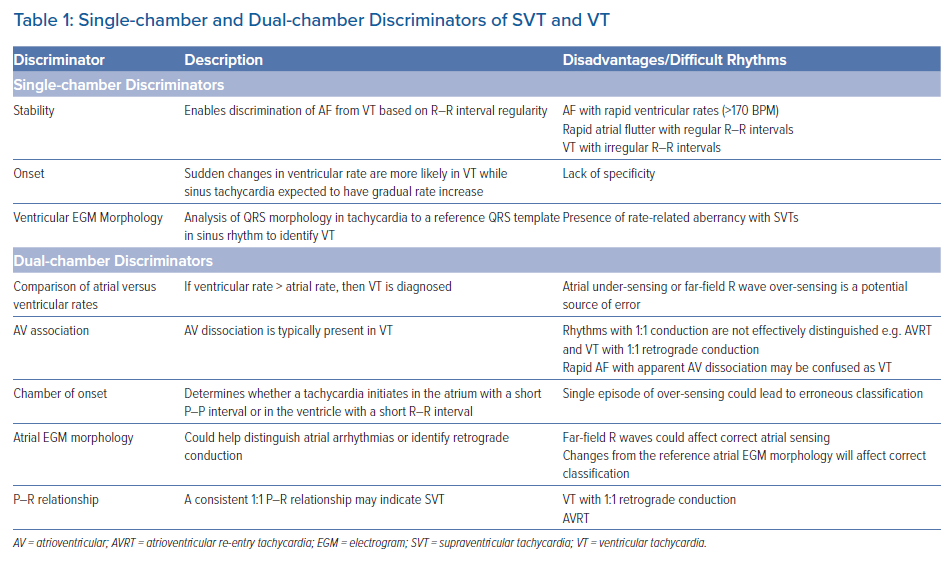
There are three main criteria used by single-chamber ICDs to differentiate SVT from VT: stability, onset and morphology. These are summarised in Table 1. The stability (R–R interval regularity) of a tachycardia allows discrimination between VT and AF. This is particularly effective when the ventricular rate is ≤170 BPM and in the absence of anti-arrhythmic drugs. However, at faster cycle lengths the R–R intervals in AF may be more regular, although the use of anti-arrhythmic medications could cause regular monomorphic VT to have greater cycle length variability or cause rapid polymorphic VT to slow down into a detection zone from a therapy zone.13 In a cohort of patients with both ischaemic and non-ischaemic cardiomyopathy, programming of a stability value of 30 ms resulted in a sensitivity of 82.7% and specificity of 91.4% for the detection of slow VT (<150 BPM), but for arrhythmia episodes >190 BPM this stability value gave a sensitivity of 78.2% and a specificity of 41.0% for detection of VT.14 There are variations in how different manufacturers calculate stability (e.g. Medtronic, 40–50 ms; St Jude/Abbott, 80 ms; Boston Scientific, 24–40 ms) and the specificity for the detection of VT can vary between 77% and 88%.15 The stability discriminator is generally used in conjunction with the other discriminators described here to achieve optimal diagnostic performance.16 An example of a device EGM of sustained VT with regular R–R intervals is shown in Figure 1.
The onset criterion enables differentiation of VT from sinus tachycardia. VT tends to initiate with a sudden onset while in sinus tachycardia the rate usually increases gradually. A potential caveat is that exercise-induced VT may be preceded by sinus tachycardia and have a gradual onset, which may lead to erroneous classification using the onset criterion. Analysis of ventricular EGM morphology has also been used to successfully discriminate between SVT and VT. The EGM source used for discrimination can be programmed to different available vectors. For example, the Wavelet morphology discrimination algorithm (Medtronic) can be programmable to one of six available vectors with the right ventricular (RV) coil–can as the nominal and RV tip–RV ring, RV tip–RV coil, superior vena cava (SVC) coil–RV coil, RV ring–can and SVC coil–can available as alternatives. A template is collected to analyse the QRS in sinus rhythm. If the ventricular rate exceeds the programmed detection cut-off, the tachycardia EGM is matched to the template. The morphological similarity of each tachycardia EGM to the sinus EGM is calculated and a resultant percentage match score derived, which is based on the differences of corresponding coefficients of their wavelet transforms.17 Using this algorithm as a single discriminator, inappropriate therapies for SVT were reduced by 78% while the sensitivity for sustained VT was 98.6%.17
The MorphMatch algorithm is used in Biotronic ICDs and is based on a beat-to-beat analysis of the QRS peak amplitude, QRS area and four major QRS deflections. QRS morphology is analysed in a 250-ms window around the peak of the QRS in the far-field EGM and the relative difference to a reference QRS is calculated for each beat. The reference QRS is continuously updated with normal beats and the mean variability of normal beats is calculated. The mean variability with the addition of a safety margin is used to program a threshold that can be used to declare a QRS complex as abnormal if the threshold is exceeded.18
Boston Scientific ICDs use the vector and timing correlation algorithm, which compares the EGM signals of a stored reference template of normal sinus rhythm to an ongoing arrhythmia. Conduction vector analysis is performed of the far-field shock EGM and the local bipolar rate EGM, and inhibition of therapy occurs if analysis of QRS morphology at eight points in each EGM matches the QRS in sinus rhythm.19
Contemporary devices manufactured by Abbott use the Far Field MD Morphology discrimination algorithm that applies the far-field vector (e.g. RV tip–can) and near-field vector (e.g. RV tip–RV ring) for alignment and correlation of the far-field QRS complex with the template. The template may be updated every 8 hours. A percentage match score is assigned and a nominal setting usually programmed whereby at least three out of ten QRS complexes have to match the morphology of the template by at least 90%.20
Both patient-related or algorithm-related factors can lead to inappropriate detection of VT using the morphology criterion, with under-detection reported in up to 2% of VTs.13 Algorithm-related factors that may prevent the accurate detection of VT using the morphology criterion include misalignment of the sinus template EGM with the tachycardia EGM, shock-induced EGM changes, signal clipping caused by incorrect signal filtering leading to EGM truncation, or EGM distortions caused by myopotentials.13,15 A rate-related bundle branch block during SVT is an example of a patient-related factor that could inhibit the performance of the morphology criterion for accurate detection. Errors due to alignment or truncation issues could be prevented through programming steps including appropriate EGM source selection and gain adjustment. Morphology discrimination is also not applied during re-detection after a shock, in order to prevent misclassification.15
Dual-chamber Discriminators
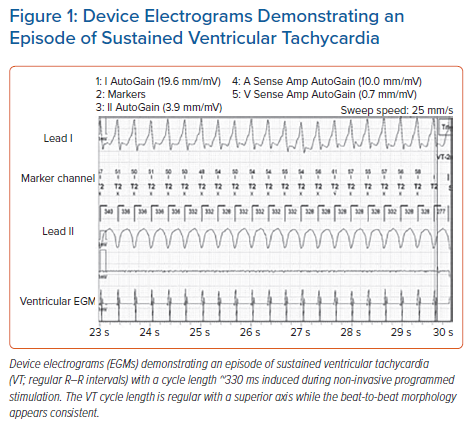
The addition of an atrial lead can increase the accuracy of rhythm identification given that additional parameters can be used in detection algorithms. These are also further summarised in Table 1. Broadly speaking, comparison of atrial and ventricular rates is a powerful discriminator because VT can be diagnosed if the ventricular rate exceeds the atrial rate (Figure 2). The AV association can also be used to distinguish VT from SVT given that VT is expected to demonstrate AV dissociation. However, this criterion does not distinguish between SVT and VT with 1:1 ventriculo-atrial (VA) conduction. In these settings, a chamber of onset criterion can be applied in which atrial tachycardia would be expected to be initiated with a short P–P interval while VT may initiate with a short R–R interval. This discriminator, however, is therefore prone to error if there is a single episode of under- or over-sensing. The single-chamber discriminators (that is, stability, onset, morphology) can also be used in conjunction, as part of the dual-chamber device detection algorithms, to ensure robust rhythm identification.21
Medtronic devices use a hierarchical, rule-based algorithm (PR LogicTM) based on atrial and ventricular rate and regularity, P–R pattern and their associations. Pairs of R–R intervals and their associated P waves are used to determine ‘couple codes’ whereby the number and timing of P waves is associated with a letter of the PR Logic alphabet. Different sequences of couple codes are associated with the various SVTs and VTs.18 When the algorithm classifies a rhythm as SVT, the device will withhold detection and therapy. Boston Scientific dual-chamber devices use the Rhythm IDTM classification algorithm, which uses the comparison of atrial and ventricular rates in addition to the vector and timing correlation algorithm described earlier.19
Biotronik uses the SMARTTM algorithm for dual-chamber detection. The first criterion is to determine whether the atrial and ventricular rate are different from each other, followed by calculation of stability on a beat-by-beat basis. The sudden onset criterion can then be applied to discriminate VT from slow-onset sinus tachycardia. Additional parameters for the algorithm include AV trend, whereby the strict order of AV intervals to identify consistent trends towards AV shortening or AV prolongation can be used to characterise AV dissociation. AV regularity and AV multiplicity (e.g. if the mean ventricular interval is a multiple of the mean atrial interval) can help establish whether atrial flutter (with 2:1, 3:1 conduction etc.) is present.18
Abbott dual-chamber devices uses a rate branch algorithm together with other discriminators such as chamber onset, AV association, AV interval delta, stability and morphology match to classify a rhythm as SVT or VT. Rate branch compares the mean atrial and ventricular intervals to identify any differences, and if no difference is found then sinus rhythm is assumed. AV interval delta sets the maximum difference between the second longest and second shortest AV interval in the QRS complexes found during the detection period. If there is a conflict between the AV association and AV interval delta algorithms, the diagnostic criteria can be programmed to resolve the scenario (e.g. an event can be adjudicated based on either discriminator classifying VT).20
There is some evidence that the addition of an atrial lead may have long-term benefits. The Dual Chamber and Atrial Tachyarrhythmias Adverse Events Study (DATAS) randomised patients with a conventional single-chamber ICD indication to a single-chamber ICD, dual-chamber ICD or a dual-chamber ICD programmed as a single-chamber ICD. The primary endpoint was a composite of all-cause mortality, invasive intervention, cardiovascular-related hospitalisation, inappropriate shock and sustained symptomatic AT lasting >48 hours. The primary endpoint was significantly lower in the dual-chamber ICD group (OR 0.31; 95% CI [0.14–0.67]; p=0.0028). There was also a reduced mortality in the dual-chamber ICD group.22 In patients with a dual-chamber ICD, improved rhythm interpretation could be facilitated at follow-up with the presence of an atrial lead, although a proportion of patients may require atrial pacing at a later date, which would necessitate a second procedure if only a single-chamber ICD had been implanted at the index procedure.22
Propensity-score matched analysis of registry data in primary prevention ICD recipients has also suggested that dual-chamber devices have a lower rate of inappropriate shock compared with single-chamber devices.23 In that analysis, independent predictors of inappropriate shock included a history of AF, chronic kidney disease and non-ischaemic cardiomyopathy, while PR Logic was the only discriminator that was related to a lower risk of inappropriate shock along with an SVT rate limit programmed to >200 BPM.23
The routine use of an additional atrial lead, however, comes at additional expense and procedural risk. In the US, the cost of a single-chamber ICD implant is approximately US$2,000 cheaper than a dual-chamber ICD.24 Single-chamber ICD patients may also require fewer follow-up procedures for complications, such as lead displacement, perforation, pneumothorax and infection. In a randomised trial using optimal programming in primary prevention patients, the routine use of an atrial lead increased procedural expense while having little impact on the frequency of inappropriate shock or quality of life at 1 year.24
Rhythm Discrimination in the Subcutaneous ICD
The placement of an extrathoracic subcutaneous ICD (S-ICD) avoids the need to enter the heart and vasculature and can be used to provide ICD therapy in patients for whom lead-related complications may be a concern and there is no pacing indication. An S-ICD uses three sensing vectors to record EGMs, which may closely resemble the surface ECG. The lead itself has two electrodes and the can serves as an electrode to result in three sense vectors. During the initial implantation, the sense vector with the optimal signal quality is selected and programmed. Rhythm discrimination is based on analysis of morphology of the S-ICD signal. During each detection, the waveform is compared with either a stored template obtained in normal sinus rhythm (static template) or with a previously detected waveform (dynamic template). These techniques can sometimes lead to the S-ICD being vulnerable to over-sensing (e.g. T wave over-sensing). The S-ICD is programmed to contain a shock zone and a conditional shock zone. The decision regarding therapy is usually based on rate alone in the shock zone while the morphology discriminators are applied in the conditional shock zone.15,25
The performance of S-ICD systems has previously been evaluated in the Subcutaneous versus Transvenous Arrhythmia Recognition Testing (START) study, in which arrhythmias were induced in patients with both S-ICDs and transvenous ICDs and the sensitivity and specificity of SVT–VT discriminators evaluated. Both ICD systems reported excellent detection of VT/VF episodes, while the S-ICD had improved specificity in the accurate classification of SVTs. This may have been due to the use of a far-field EGM source for the S-ICD, which provides greater information on global cardiac activation, as well as to the increased number of analysis points for each ventricular complex in the S-ICD (i.e. a higher resolution analysis of the signal).26 New discrimination algorithms to reduce T wave over-sensing have also been implemented to reduce inappropriate shock using a correlation of existing complexes to previous complexes.27 Recently, the multi-centre PRAETORIAN trial reported that in patients without a pacing indication, an S-ICD was non-inferior to the transvenous ICD with respect to device-related complications and inappropriate shock, with no difference in appropriate shock between the two arms.28
Challenging Arrhythmias
Tachycardias that have a stable 1:1 AV relationship (e.g. AVNRT or VT with 1:1 retrograde conduction) may be difficult to differentiate using the stability and AV association criteria. In these circumstances, the chamber of onset criterion may be able to distinguish VT from an atrial tachycardia with rapid onset. Morphology may also be used for discrimination. AVNRTs typically have a 1:1 AV relationship with almost simultaneous atrial and ventricular depolarisation, with a VA interval <70 ms. The onset may be spontaneous and triggered by a premature atrial contraction and there may be a good match to the reference QRS morphology. AVNRT is a frequent cause of inappropriate ICD therapy given that the arrhythmia often conducts rapidly (>200 BPM), falling into the programmed VT or VF zones. Atrial activation may also occur in the PVAB period, which may lead to under-sensing of the atrial EGM and lead to incorrect classification of the rhythm as VT (given that tachycardia is classified as ventricular rate > atrial rate and therefore therapy will be delivered).29
The response of the tachycardia to unsuccessful ATP can also be helpful in the differential diagnosis. An atrial tachycardia may terminate with ATP if the atrial rate is accelerated and resets the tachycardia circuit, while ventricular overdrive pacing may also terminate AVNRT. This response is not helpful in differentiation because VT may also terminate with overdrive pacing. However, if the tachycardia is entrained during ATP, the post-pacing response can help elucidate the mechanism, for example, in the case of a tachycardia with a 1:1 AV relationship that has not been successfully terminated by ATP and instead the atrial rate has been accelerated. Similar to a diagnostic electrophysiology study, an A-A-V response following cessation of pacing would rule in an atrial tachycardia, while a V-V-A response would be diagnostic of VT.15 A short post-pacing interval minus tachycardia cycle length (PPI − TCL <30 ms) during ventricular overdrive pacing would also favour VT or an AVRT over AVNRT, given that this suggests that the ICD lead is in close proximity to the circuit.29,30 Occasionally, VA block may be seen during ATP, without termination of the tachycardia, which would favour VT.31 However, this response does not definitively exclude AVNRT with retrograde block.
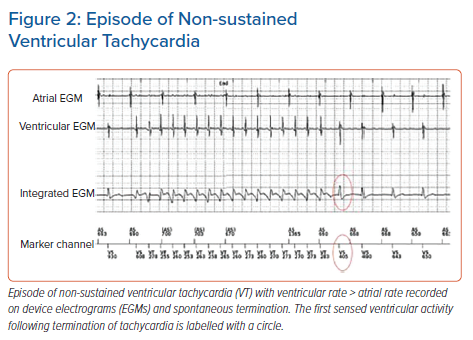
The differentiation between AF and VF is usually made using the stability, AV association and morphology criteria. However, misclassification can occur if AF is particularly rapid and there is normalisation of the R–R intervals or if aberrancy with bundle branch block is present, which may make these discriminators unreliable. Inappropriate shock for AF or atrial flutter can be associated with an increased overall risk of mortality, particularly in younger patients (Figure 3).32
Despite the use of ICDs in the primary and secondary prevention of sudden cardiac death, all-cause mortality remains high in ICD recipients over the medium to long term. In a large retrospective analysis of cause of death in >2,700 ICD recipients, congestive cardiac failure accounted for 30% of all deaths while sudden death occurred in 20% over a mean follow-up of 5.4 years.33 Based on available post-mortem interrogations, >50% of sudden deaths in this cohort occurred due to ventricular arrhythmias despite the presence of an ICD, which may have been due to inadequate recovery of myocardial output due to the underlying cardiovascular pathology, an acute myocardial ischaemic event or the presence of electromechanical dissociation.33
Conclusion
There are a number of single-chamber and dual-chamber discriminators that can help distinguish SVT from VT and ensure that ICD therapies are applied appropriately. These discriminators are integrated into different algorithms that are used by the various device manufacturers. The performance of these discriminators is reliant on accurate atrial and ventricular sensing. In patients without a pacing indication, an S-ICD may be an option and has shown similar rates of device-related complications and inappropriate shock as a transvenous ICD, while avoiding the complications related to lead insertion and long-term lead-related complications. Despite these capabilities, the presence of SVTs remains a leading cause of inappropriate therapy in ICD recipients.
Clinical Perspective
- The majority of inappropriate shocks in patients with an ICD are caused by supraventricular tachycardias such as AF, atrial flutter, atrial tachycardia, atrioventricular (AV) re-entry tachycardia and AV nodal re-entry tachycardia (AVNRT).
- Modern pacing and defibrillator systems have a number of algorithms to enable reliable discrimination of supraventricular tachycardia from ventricular tachycardia, in order to limit therapies to those rhythms that definitively require them.
- It can be challenging to discriminate between tachycardias with a stable 1:1 AV relationship, for example ventricular tachycardia with 1:1 retrograde conduction or rapid AVNRT with a cycle length <300 ms, the latter of which may be a frequent cause for inappropriate ICD therapy.