Philippe Coumel, one of the founding fathers of modern arrhythmology, stated in 1978: “There are always three main ingredients required for the emergence of a clinical arrhythmia: an arrhythmogenic substrate, a trigger and modulating factors such as imbalanced autonomic nervous tone and electrolyte disorders.”
The basic understanding of the mechanisms underlying ventricular arrhythmia (VA) is essentially unchanged. It is generally accepted that the occurrence of VA in ischaemic cardiomyopathy is an interplay between the presence of fibrotic scar tissue and transient electrical instability creating an arrhythmogenic milieu. An electrical unstable environment may be caused by an ischaemic event, autonomic imbalance, electrolyte disturbances, increased wall stress in heart failure patients or a combination of factors.1 The potentially lethal combination of these sudden cardiac death (SCD) risk factors is often unique to the individual and may change over time. The latter explains why there are still no optimal risk models available to identify patients with an increased risk of SCD. After all, one SCD that could have been prevented is one too many.
Results of intensive research in the past 20 years have led to an increase in ICD implantations worldwide, of which primary prevention is the most common indication today. In general, the rationale behind secondary prevention ICD implantation is clear and there is no discussion regarding the indication for ICD eligibility in SCD survivors or patients with previous sustained VA. However, selecting patients for primary prevention ICD implantation is a far more complex matter and requires a meticulous decision-making process, with individual risk assessment and counselling. The risks and benefits of ICD implantation need to be discussed based on medical considerations and patients’ personal preferences. Current guidelines recommend implantation of a prophylactic ICD in patients with functional New York Heart Association class I or II–III and left ventricular ejection fraction (LVEF) ≤30–35% at least 40 days post-MI and in US guidelines a condition of 90 days post-revascularisation.2,3 Real world registry data show that only a minority of patients selected for primary prevention ICD implantation based on current guidelines benefit from the ICD because of a low incidence of appropriate ICD therapy.4 An Israeli registry documented only 2.6% appropriate ICD therapy in patients with a prophylactic ICD (>70.0% ischaemic cardiomyopathy) after 2.5 years of follow-up.5 Furthermore, device and lead-related complications and inappropriate ICD shocks are important disadvantages of ICDs, which can have a significant negative impact on patients’ quality of life.6,7
In this review, we highlight the gaps of knowledge in the available literature on primary prevention ICDs and the additional value of the Defibrillator After Primary Angioplasty (DAPA) trial results.8 We also discuss the potential role of contemporary advanced imaging modalities in risk stratification of patients for prophylactic ICD implantation.
Primary Prevention ICD, Ageing Literature?
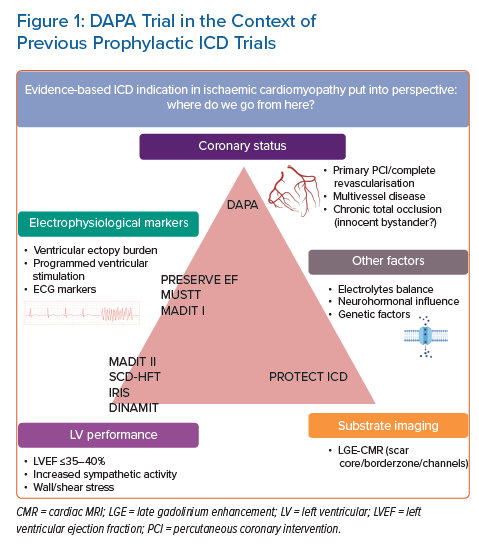
One of the most important cornerstones of today’s primary prevention ICD implantation guidelines for ischaemic cardiomyopathy is based on the results of MADIT II in 2002.9 Patients were included based on a history of remote MI (mean 6.7 years after index MI) and LVEF (<30%). Patients who underwent revascularisation within 3 months were excluded from the analysis. This complicates translation of MADIT II to today’s practice, as it is unknown whether outcomes are similar in ST-elevation MI (STEMI) patients who are treated with primary PCI. The MADIT II population was a heterogeneous population from the perspective of Coumel’s triangle of factors involved in arrhythmic SCD. It is unknown whether two hypothetical post-MI patients with comparable LVEF <30% and either: a relatively small myocardial scar and suboptimal revascularised multivessel coronary disease or large MI after a successful revascularisation of a single vessel coronary artery disease, bear the same risks for VA/SCD.
Of note, there is increasing evidence that infarct-related chronic total occlusions may contribute to an enhanced susceptibility for VA in both primary and secondary prevention.10 Moreover, new advances in late gadolinium enhancement (LGE) cardiac magnetic imaging (CMR) allows a detailed scar characterisation and has potentially promising value in the refinement of risk stratification and prediction of VA/SCD.11,12 The shortcomings of LVEF as a sole risk stratification factor for post-MI primary ICD indication are clear and it has been criticised numerous times, yet alternatives are still lacking.13–15
A recent retrospective cohort analysis of four MADIT studies addressed the most important question in qualification of primary prevention ICD benefit: risk of life-threatening VA against competing risk of non-arrhythmic mortality as a result of pump failure.16 Patients with the greatest ICD benefit were considered to have an increased 3-year predicted risk of VA and a relatively low predicted 3-year risk for non-arrhythmic mortality, leading to a 74-days life gain in 3 years. A high VA score included established factors such as younger age, prior non-sustained ventricular tachycardia, faster baseline heart rate (increased sympathetic activity) and prior MI. While these factors may contribute to an improved risk stratification scheme, primary prevention trials (MUSTT, SCD-HeFT) using a variability of these criteria, had limited impact on the guidelines.17,18 The mixture of inclusion criteria, different primary outcomes (all-cause mortality or arrhythmic mortality) and patient populations (including non-ischaemic cardiomyopathy) potentially made interpretation of the available data even more complicated.19
Furthermore, preventive measures to reduce the burden of coronary artery disease and heart failure have significantly improved over the past decades and their impact on the incidence of VA/SCD is not fully delineated yet. In conclusion, existing data in the area of primary ICD implantation seem to be outdated and are becoming more and more estranged from contemporary cardiology practice.20 However, repeating the previous clinical landmark trials, randomising patients with LVEF <30% to ICD implantation compared with optimal medical therapy, would now create an ethical dilemma. Despite promising results of novel heart failure drugs (for example, angiotensin receptor-neprilysin inhibitors), withholding an ICD from patients with LVEF <30% would be difficult to defend, especially in younger patients.21 Currently, researchers are challenged to develop novel risk stratification models based on observational and retrospective data in patients with severely reduced LVEF.22
DAPA Trial and the Timing of Implantation
The latest randomised trial that compared ICD implantation with optimal medical therapy only in patients with LVEF <30% was the DAPA trial.8 This randomised, multicentre, controlled trial was conducted in high-risk primary PCI patients, defined as either:
- LVEF <30% (being the only criterion in 66.5% of the participants);
- failure or suboptimal result of acute revascularisation (TIMI flow less than three in 20.3% of the participants); or
- a combination of factors, including primary VF and Killip class greater than −2.
The primary endpoint was all-cause mortality after 3 years. Additional survival assessment was performed in February 2019 for the primary endpoint. The trial prematurely ended after inclusion of 266 patients as a result of a slow inclusion rate (38% of the calculated sample size) with 131 patients randomised to the ICD arm and 135 patients to the control arm. The greatest differences from former trials is that every patient underwent primary PCI (according to current standard of care) and echocardiographic LVEF evaluation was performed within 4 days of STEMI, in contrast to persistent low LVEF after remote MI such as in MADIT II, in which ICD implantation was performed approximately 6.7 years after index MI.
Although not confirmed by CMR-LGE imaging, low LVEF early after primary PCI in the DAPA trial may be regarded as a surrogate for a large scar caused by the acute MI (mainly anterior infarctions). Another important difference is that ICD implantation in the DAPA trial was postponed to at least 30 days post-MI (median of 50 days) to overcome the issue of mortality related to heart failure in the early healing phase, as this was the pitfall that caused the negative outcomes of IRIS and DINAMIT.23,24 Several issues related to the DAPA trial, of which premature termination is the most important factor, limit the ability to draw strong conclusions as justly expressed by Parkash et al.25 However, some interesting findings need to be recognised and may be used as a guide for future research.
DAPA data show a beneficial impact of early ICD implantation on all-cause mortality within the first 3 years after MI and this was sustained at 9 years of follow-up. In both groups, patients had similar follow-up visits. As such, we consider the potential bias of an improved heart failure treatment regime in the ICD group unlikely. It is reassuring that ICD implantation in low LVEF post-STEMI patients is associated with improved survival in this particular cohort of patients with STEMI treated with primary PCI, which is in line with the results of MADIT II. Nevertheless, it is important to realise that MADIT II focused on ICD benefit in patients with long-term low LVEF in general, while the scope of the DAPA trial is limited to patients in the current era with the widespread use of primary PCI. Different scopes of previous and future ICD trials are shown in Figure 1.
A subanalysis of MADIT II revealed that in patients with recent revascularisation (less than 6 months previously), there was no benefit of ICD implantation.26 In contrast, the DAPA trial shows that appropriate ICD therapy occurred in five patients (1.9%) within the first 8 months after implantation. Primary PCI of the infarct-related artery reduces mortality by reducing infarct size and diminishing myocardial scarring-related arrhythmogenicity.27 However, several questions remain unanswered: Is arrhythmic SCD in the early healing phase after MI caused by a complex interaction between autonomic imbalance, triggering factors and scar inhomogeneity? Or does the extent of scar burden become more relevant over time after the early remodelling phase?
The following factors are important contributors to VA/SCD risk in the early phase after MI: success of revascularisation, severity of LV dysfunction, electrical instability and wall stress. Although we know the common denominator in the DAPA trial was a large infarction with severe LV dysfunction in the early phase after STEMI, the subgroup of patients with VA was too small to identify true triggers for VA. Another interesting finding of the DAPA trial is that follow-up data suggest the benefit of ICD may extend to patients whose LVEF had improved over time. LVEF increased ≥10.0% in 46.5% of the patients during follow-up. In a subgroup analysis of all patients with LVEF ≥35% (n=117) after 18 months of follow-up, there was a trend towards ICD benefit (HR 0.34; 95% CI [0.09–1.25]; p=0.100). Although these analyses are exploratory, similar results were found in a subanalysis of the SCD-HeFT.28 Furthermore, the VALIANT trial has shown that baseline LVEF <40% was strongly associated with the occurrence of SCD in hospital and after discharge and explained a limited amount of SCD risk during long-term follow-up (longer than 6 months).29
To conclude, the DAPA trial has several limitations and lacks potentially important data regarding coronary revascularisation during follow-up and novel medical treatment strategies. Nevertheless, it is unique in its design compared with other primary prevention trials as patients all underwent primary PCI, an essential part of today’s treatment strategy in acute MI patients. Pharmacological and cardiac resynchronisation trials have shown that neurohormonal antagonists (for example angiotensin receptor–neprilysin inhibitors) and cardiac resynchronisation therapy (CRT) have a significant impact on heart failure hospitalisation and heart failure related death, but the effect on SCD is not well appreciated.30 As the journey of advancements in ICD therapy for primary prevention will continue, there is a clear need for future trials focusing on SCD risk assessment based on advanced substrate characterisation combined with contemporary coronary revascularisation and pharmacological therapy.
The Value of Cardiac MRI
Although scar characterisation with LGE-CMR imaging is gaining interest, it still has several limitations. Solid data regarding the added value of substrate imaging for a more tailored and robust risk stratification of SCD in reduced LVEF patients are lacking.31 The occurrence of VA/SCD does not solely depend on the size or other characteristics of the substrate. The susceptibility for VA/SCD is based on a complex interplay of multiple factors.32,33 Furthermore, there is currently no consensus on which CMR method should be used to identify patients at risk for VA.34,35 Various observational studies indicated that the peri-infarct zone assessed by CMR is a strong predictor of all-cause mortality and arrhythmic outcomes in ischaemic cardiomyopathy in both severely depressed and moderately depressed LV function and even CRT patients.33,36 However, there is significant heterogeneity between studies regarding type and dose of contrast agent administration, CMR sequence and assessment of border zones.37,38
Also, timing of CMR after MI was not reported in most studies. As such, data regarding the importance of temporal dynamics of scar aging on VA risk are limited.35,39 The reason why young patients may benefit most from ICD may be the result of fewer competing risks of death.40 However, the impact of the dynamic nature of the scar healing process irrespective of patient age is not fully delineated yet.41,42 First presentation of VA/SCD may occur 10 years after the index MI, which younger patients are more likely to experience. Whether this is caused by a triggering factor (for example, acute ischaemia in progressed coronary disease), heart failure or a chronic myocardial scar, is unknown.
Future research with CMR imaging at different time points after MI might provide more insights into the dynamic changes in the myocardial substrate. It was recently shown that the absence of conduction channels in the scar tissue and limited scar mass (<10 g) can identify patients at a very low risk of VAs in a primary prevention population with a negative predictive value of 97.2%.43 Implementation of advanced imaging in the standard follow-up of these patients may allow discrimination of low risk groups as a first step in refining primary prevention ICD indications, or perhaps even broaden ICD indications in patients with LVEF >35% and LGE presence, a population currently investigated in the CMR GUIDE trial.44
Risk Stratification Beyond Left Ventricular Ejection Fraction and Substrate
The results of the PRESERVE EF trial indicate that non-invasive risk factors,for example premature ventricular complexes, increased T wave alternans, reduced heart rate variability, etc., are important warning signals for the risk of VA in post-MI patients, even in preserved LVEF. The results also underline that programmed ventricular stimulation (PVS) may be useful to identify patients at risk for VA in selected cases, as was previously shown in randomised trials, for example MUSTT and MADIT.45–48 However, patients in the early post-MI period were largely excluded from the above mentioned randomised controlled trials. The PROTECT ICD trial, an international, multicentre randomised controlled trial uses a PVS protocol to discriminate patients with LVEF <40%, early post-MI who will potentially benefit most from an ICD.49 The combination of CMR imaging and PVS in early post-MI patients (2–40 days), has the potential to fill an important knowledge gap in the landscape of primary prevention ICD implantation indication.
Conclusion
Robust and sophisticated SCD risk stratification in ischaemic cardiomyopathy patients in general, and STEMI patients treated with primary PCI in particular, remains a demanding challenge, as a combination of dynamic factors (e.g. heart failure, coronary revascularisation status and myocardial substrate characteristics) are involved in the susceptibility to SCD. Current guidelines need more nuance based on altered SCD risk levels as a result of contemporary pharmacological and non-pharmacological treatment strategies. The DAPA trial results suggest that the extent of LV dysfunction shortly after STEMI that is treated with primary PCI can be used to identify patients at enhanced risk for early mortality. However, similarly to other characteristics, the ability to predict clinical events may be substantially less after a certain critical time window. Advanced substrate imaging with LGE-CMR has the potential to embrace an important factor that may guide clinicians in providing tailored therapies to decrease disease burden and increase survival, but we are not there yet. With respect to previous landmark primary prevention trials, updated randomised trials (including invasive and non-invasive tests) are needed with the inclusion of a contemporary population, treated with novel treatment strategies.
Clinical Perspective
- Current guidelines for primary prevention ICD eligibility are mainly based on left ventricular ejection fraction (LVEF) ≤30–35%.
- The shortcomings of LVEF as a sole risk stratification factor for post-MI primary ICD indication are clear, yet alternatives are lacking.
- Despite limited power, the DAPA trial conveys important messages and provides novel perspectives regarding left ventricular function post-primary percutaneous coronary intervention as an early risk marker for sudden cardiac death.
- New randomised trials are needed that include a contemporary population, treated with novel treatment strategies.