The obesity epidemic continues its relentless advance, currently affecting 2 billion people.1 Obesity is associated with an increased burden of a spectrum of diseases, with the focus in this article being on AF. The health-related burden associated with obesity is estimated to have a substantial economic impact.2 In alignment with this epidemic, the prevalence of AF is increasing. In the US, a large observational study showed a significant increase in the incidence of new AF between 1980 and 2000, and estimated that the projected number of persons with AF in the US would exceed 10 million by 2050.3 Similar trends have been seen in Europe and Australia.4,5 The pathophysiological mechanisms that underline the AF–obesity relationship remain incompletely understood. There are, however, consistent data that support weight management in improving AF outcomes. In this review, we discuss the AF–obesity relationship, the proposed underlying mechanisms, and the impact of weight loss on AF and its arrhythmogenic substrate.
The Obesity–AF Association
A number of observational cohort studies have highlighted obesity as an independent risk factor for the development of AF.6–11 In the Framingham Heart Study, 526 out of 5,282 participants developed AF over a mean follow-up of 13.7 years.11 Obesity was found to be an independent predictor for incident AF after adjusting for traditional risk factors. Similar findings were found in the Women’s Heart Study, where incident AF was confirmed in 2.4% of the cohort over a 12.9-year follow-up period.9 BMI was linearly associated with AF risk, with a 4.7% increase in risk with each kg/m2.
To examine the impact of the obesity-associated traditional risk factors on the AF risk, a recent retrospective Korean study studied the AF risk among a cohort of metabolically healthy obese individuals (no diabetes, hypertension and dyslipidaemia) compared with metabolically unhealthy obese and non-obese individuals.8 Compared with non-obese individuals, the metabolically healthy obese cohort had a 20% increased AF risk, whereas metabolic unhealthiness increased the AF risk by 40%. In addition, elevated BMI has been shown to be associated with AF clinical phenotype progression from paroxysmal to persistent.10 In the Olmsted County study, where 3,248 patients with paroxysmal AF were followed up, 17% progressed to persistent AF. Obesity was independently associated with progression to persistent AF, even after adjusting for traditional risk factors, signalling its impact on the arrhythmogenic substrate of AF.
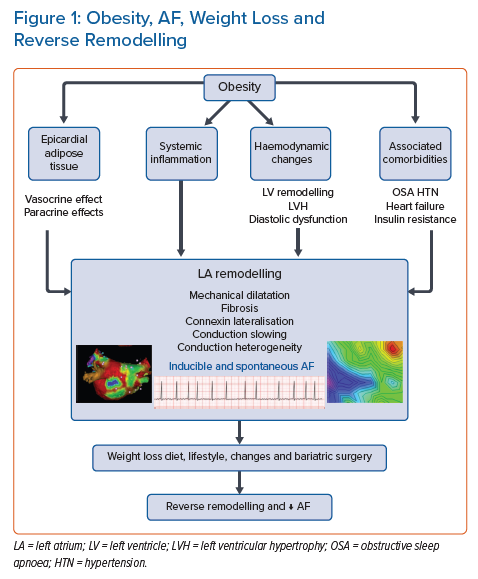
However, while the aforementioned evidence supports the obesity–AF relationship, the mechanism underpinning the impact of obesity on the incidence of AF remains incompletely understood and is likely multifactorial (Figure 1). In the next section, we discuss the potential mechanisms that underpin the obesity–AF relationship, highlighting the recent insights from both animal and human studies.
Pathophysiological Changes in Obesity and AF
Haemodynamic Changes
Obesity leads to left atrial and ventricular remodelling through a number of haemodynamic mechanisms. Obesity is associated with an increase in both cardiac output and systolic blood pressure with little change in the heart rate.12 This is related to both activation of the renin–angiotensin–aldosterone system and enhancement of the sympathetic nervous system.13 This increase in cardiac output will increase venous return to the heart, leading to enhanced atrial and ventricular wall stretch, and eventually dilatation.14 Moreover, obesity has a haemodynamic impact on the heart through the associated medical comorbidities, such as hypertension, sleep apnoea and insulin resistance. Hypertension increases left ventricular afterload, leading to further structural remodelling in the form of left ventricular hypertrophy, diastolic dysfunction and left atrial dilatation.15 Obesity-associated sleep-disordered breathing is associated with surges in symptomatic nervous system activity through intermittent episodes of hypoxia, which has been shown to be associated with a significant increase in the prevalence of both atrial and ventricular arrhythmia, even after adjusting for traditional risk factors.16
Left Atrial Remodelling and Left Atrial Substrate
Animal studies have consistently demonstrated the presence of an arrhythmogenic substrate in association with obesity. In an ovine model, progressive changes in electrical and structural atrial remodelling were seen in a cohort of 30 sheep being fed a high-calorie diet over an 8-month period.17 Increasing weight was associated with increasing left atrial (LA) volume, LA fibrosis and upregulation of inflammatory markers. The net effect was decreased conduction velocity and an increase in conduction heterogeneity, with an associated increase in both inducible and spontaneous AF. Other animal studies noted that a high-fat diet could increase AF duration due to slow atrial conduction and reduced pulmonary vein refractoriness. These changes could occur without necessarily being accompanied by development of obesity.18,19
Similar abnormal substrate has been seen in human studies. A large observational longitudinal study found that obesity was a strong predictor of LA enlargement, after adjusting for age and sex.5 Moreover, Mahajan et al. characterised the electroanatomic atrial remodelling and epicardial adipose tissue in a cohort of obese patients and compared it with a non-obese cohort.20 Obesity was associated with an increase in all measures of epicardial adipose tissue, with a predominant distribution adjacent to the posterior left atrium and the atrioventricular groove. Obese patients had reduced global conduction velocity, increased fractionation and increased low-voltage areas. Low-voltage areas were predominantly seen in the posterior and/or inferior LA, matching the location of epicardial adipose tissue (EAT) on cardiovascular MRI.
Role of Epicardial Adipose Tissue
The availability of cross-sectional imaging modalities such as cardiac computed tomography has enabled us to examine the relationship between the quantity and quality of the EAT and AF. This layer of adipose tissue is located between the visceral pericardial and the epicardial surface, and there is no fascial layer that separates the EAT and the myocardium. Epidemiological studies utilising non-invasive imaging with a focus on the abundance of EAT have consistently demonstrated an independent association between EAT volume and incident AF, even after adjusting for AF risk factors, including BMI and LA enlargement.21,22 Beyond predicting AF, this association appears to impact on the outcome of AF ablation.23 However, the underlying electrophysiological, cellular and molecular mechanisms that link epicardial adipose tissue with AF progression remain poorly defined, and a number of mechanistic theories have been proposed.
The proximity of the EAT layer to the myocardium means the EAT can exert important paracrine and vasocrine effects on neighbouring cardiomyocytes.24,25 In chronic inflammatory disorders, the epicardium becomes a site of deranged adipogenesis, leading to secretion of pro-inflammatory adipokines, such as interleukin-1β, interleukin-6, activin-A and tumour necrosis factor-alpha, which may play an important role in the development of atrial fibrosis.26 In an ovine model, Mahajan et al. demonstrated that sustained obesity resulted in global biatrial endocardial electrical and structural remodelling, and associated EAT infiltration in the posterior LA wall.27 A cohort of 10 sheep fed a calorie-dense diet to induce obesity were compared with 10 lean sheep, and all 20 sheep underwent invasive and non-invasive assessments of their atrial substrate. Compared with the lean sheep cohort, the obese sheep demonstrated both abnormal structural (increased LA volume and pressure) and electrical (reduced atrial conduction velocity, increased conduction heterogeneity, increased fractionated electrograms and decreased posterior LA voltage) remodelling. This was associated with more frequent, prolonged and greater cumulative duration of AF. Epicardial fat was seen to infiltrate the posterior LA in the obese group, and was associated with reduced endocardial voltage in this region.
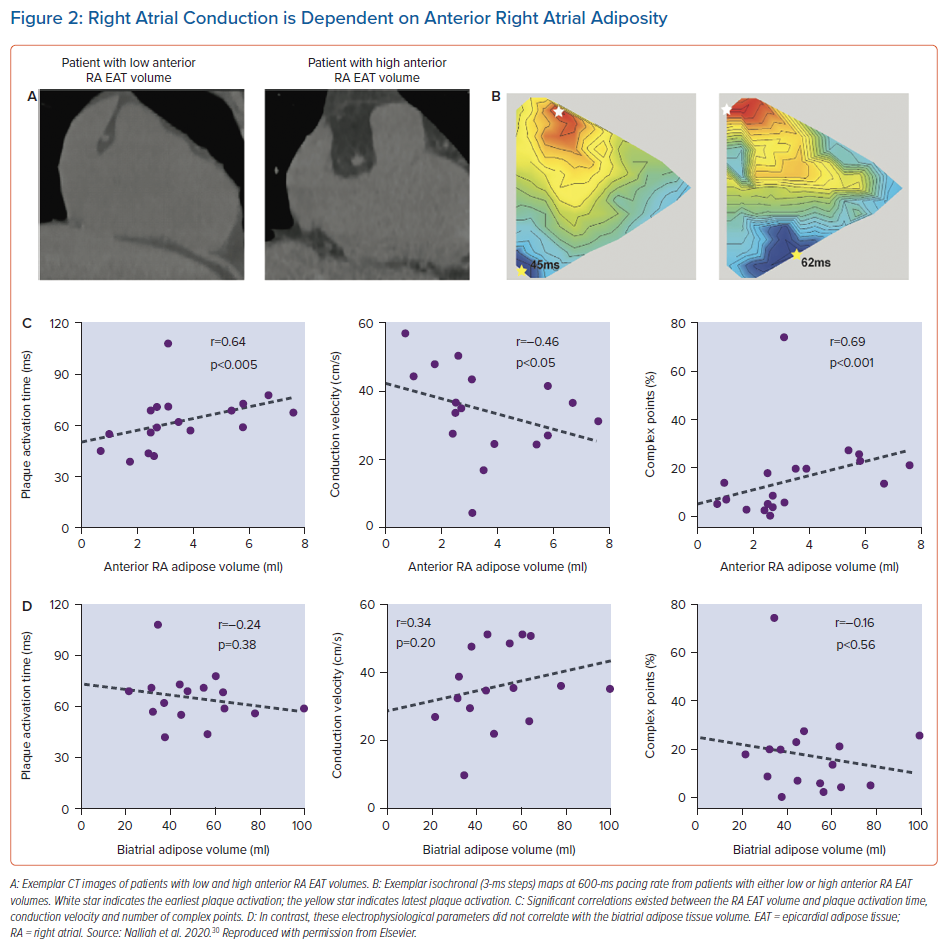
Prior tissue culture studies have shown that epicardial adipose tissue is capable of releasing adipo-fibrokines, promoting atrial fibrosis and its associated electrical substrate.28,29 The critical role local EAT has on the atrial substrate was elegantly demonstrated in a recent study.30 In a cohort of patients undergoing cardiac surgery without AF, higher local epicardial adipose tissue volume on imaging correlated with slowed conduction, greater electrogram fractionation, increased fibrosis and lateralisation of cardiomyocyte connexin-40 (Figure 2). Moreover, atrial conduction heterogeneity was increased with more extensive myocardial adipose infiltration. Cardiomyocyte culture studies using multielectrode arrays showed that cardiac adipose tissue-secreted factors slowed conduction velocity and contained proteins with capacity to disrupt intermyocyte electromechanical integrity.
Recently, non-invasive assessment of the EAT activity on CT has gained significant interest. As inflammation increases, a significant change in the degree of epicardial fat attenuation values represented by Hounsfield units can be detected.31 This suggests that EAT attenuation may serve as a novel non-invasive radiographic sensor of inflammation. Adipose tissue activity on CT has been shown to be an independent predictor of high-risk coronary artery plaques and major adverse cardiovascular events.32 El Mahdiui et al. demonstrated that a higher attenuation of the EAT was associated with higher AF recurrent rates.33 In a cohort of 460 patients undergoing first AF catheter ablation for symptomatic AF, the total EAT and posterior LA adipose tissue was traced, and the attenuation value of the posterior LA adipose tissue was assessed. Following a mean follow-up of 18 months, 37% patients had AF recurrence, and patients with higher attenuation (>–96.4 Hounsfield units) were more likely to have AF recurrence than lower attenuation on both univariate and multivariate analyses. Similar findings were demonstrated in another study that examined attenuation of the entire LA periatrial EAT.34 The ability of cardiac CT to provide both quantitative and qualitative assessments of the EAT provides a promising tool that may assist in individualising therapy for patients with AF.
Role of Inflammation
In addition to the local inflammatory role of EAT on the arrhythmogenic substrate of AF, obesity is associated with elevated systemic inflammatory markers, which have been linked to AF. Inflammation has been linked to various pathological processes, such as oxidative stress, apoptosis and fibrosis, which may promote AF substrate formation.35
Impact of Weight Loss on the Atrial Substrate and AF
Observational retrospective studies have suggested a role for weight loss in reducing AF.36 A number of prospective studies, both observational and randomised, have confirmed the favourable effect of weight loss on AF.37–40 Abed et al. randomised 150 patients with AF to either weight management (intervention) or general lifestyle advice (control).37 At 1 year, the intervention group lost significantly more weight compared with controls, and had fewer AF episodes and symptoms, and less burden. In addition, the LA diameter and interventricular septum wall thickness was significantly reduced compared with the control arm.
In the LEGACY study, Pathak et al. demonstrated that patients who lost and maintained the loss of >10% of their bodyweight over 4 years had sixfold arrhythmia-free likelihood compared with those who lost <3% or gained weight. Moreover, weight fluctuation of >5% had an adverse effect on overall freedom from AF, suggesting the causal relationship between weight and AF.39 In addition, compared with baseline, patients who lost >10% of bodyweight had significantly decreased left atrial volume indexed for body surface area, interventricular septum thickness, left ventricular end diastolic diameter and E/E’, whereas patients who lost <3% had either an increased or unchanged difference from baseline. It is important to note that while this study targeted weight loss, it was associated with improvements in sleep apnoea, blood pressure and glycaemic control; making it difficult to ascertain the individual impact of each risk factor on both AF reduction and reverse remodelling.
A subanalysis of the same study cohort (REVERSE-AF trial) examined the association between weight loss and progression of AF.38 When patients were stratified according to the percentage of weight loss (group 1: <3%, group 2: 3–9% and group 3: >10%), 41% progressed from paroxysmal to persistent and 26% from persistent to paroxysmal or no AF in group 1. In group 2, 32% progressed from paroxysmal to persistent and 49% reversed from persistent to paroxysmal or no AF. In group 3, 3% progressed to persistent and 88% reversed from persistent to paroxysmal or no AF (p<0.001). Moreover, increased weight loss was significantly associated with greater AF freedom: 45 (39%) in group 1, 69 (67%) in group 2 and 116 (86%) in group 3 (p<0.001). It is important to remember that the majority of the data on weight loss in AF used lifestyle changes to achieve weight loss, which without constant supervision is often unsustainable or unsuccessful.
As such, the impact of surgical weight loss through bariatric surgery on AF ablation outcomes was recently examined in an observational study by Donnellan et al.41 They reviewed a cohort of 51 morbidly obese patients (BMI >40 kg/m2) who had undergone bariatric surgery prior to AF ablation, and matched them to a cohort of 102 morbidly obese patients without prior bariatric surgery and 102 non obese patients. The three groups had similar LA dimensions on echocardiography at baseline. Bariatric surgery was associated with significant reductions in weight, systolic blood pressure and glycated haemoglobin. During follow-up, the bariatric surgery group had significantly fewer AF recurrences compared with the other two control groups.
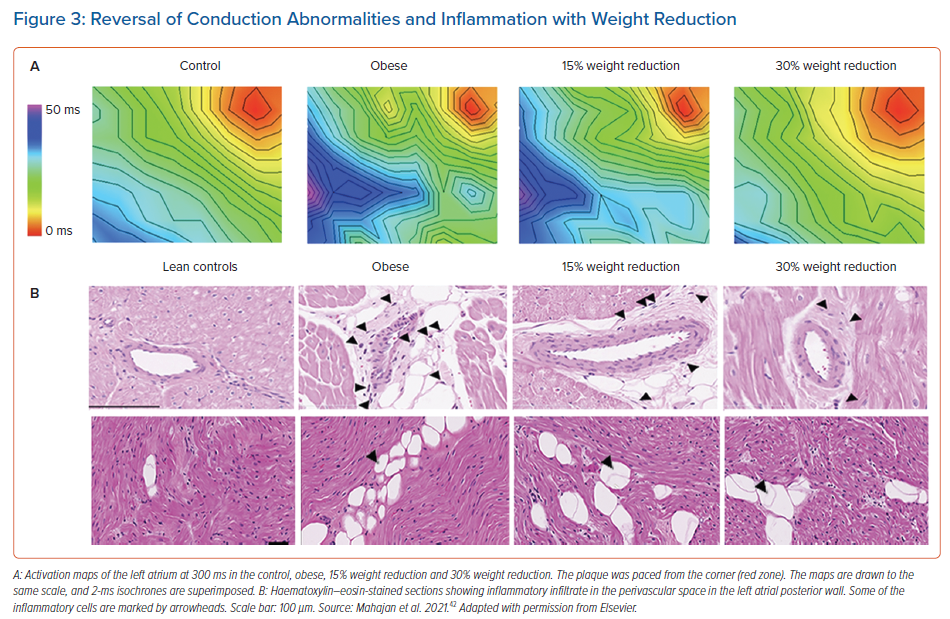
The precise mechanism by which weight loss leads to AF reduction is not well defined. Recently, Mahajan et al. studied the impact of weight reduction on reverse remodelling of the atrial electrical and structural substrate.42 In a cohort of 30 sheep that had sustained obesity induced by a high-calorie diet over 18 months, the cohort was randomised into three groups: sustained obesity, and 15% and 30% weight loss. Sustained obesity was associated with adverse LA structural and electrical remodelling, whereas 30% weight loss was associated with a significant reversal of remodelling, translating into less AF inducibility (Figure 3).
Weight Management in Obese Patients with AF
The compelling body of evidence that supports weight reduction in the management of AF is clear. This has been reflected in scientific statements from both the European Society of Cardiology and the American Heart Association.43,44 The 2020 European Society of Cardiology guidelines for management of AF proposed the Atrial fibrillation Better Care (ABC) holistic pathway (‘A’ anticoagulation; ‘B’ better symptom management; ‘C’ cardiovascular and comorbidity optimisation) as a simplified approach to care for AF patients across different health disciplines. Intense weight reduction, together with comprehensive management of interacting cardiovascular risk factors, is the cornerstone of the ‘C’ component of the ABC pathway.44
However, successful and sustainable weight reduction remains a challenging task to achieve and a number of strategies have been proposed. These strategies stem from the concept of a chronic care model, which has been successful in the management of patients with various chronic illnesses, such as ischaemic heart disease and chronic heart failure. The integrated care approach in which the patient is the primary focus, together with multidisciplinary teams and community support, has been shown to be associated with a reduction in all-cause mortality and cardiovascular hospitalisations in a recent meta-analysis.45
Sanders et al. showed that a dedicated risk factor management clinic focusing on all modifiable risk factors through both lifestyle and diet changes can deliver promising results.39,46 Such clinics have the potential to do this through identifying patient-specific modifiable risk factors, setting individual achievable targets, and working with patients to ensure ongoing motivation and adherence to their goals. Understanding the difficulties associated with achieving and maintaining weight reduction, bariatric surgery has the potential to achieve such results, and has been demonstrated to be associated with both a reduction in the incidence of AF among patients at risk and reversal of AF type among patients with known AF.47,48
Conclusion
Obesity and AF have emerged as major global epidemics. Their impact on the healthcare costs and burden on both patients and physicians is evident. There is strong evidence that supports the obesity–AF relationship through multiple and interacting mechanisms, including diastolic dysfunction, local EAT inflammation and infiltration, and systemic inflammation. The current body of evidence strongly supports weight loss as an important pillar in the management of obese patients with AF. Weight reduction together with the management of other risk factors should be delivered using a comprehensive approach to obtain the best results.
Clinical Perspective
- Obesity is an independent and important predictor of AF incidence and progression.
- Emerging data now support the impact of epicardial adipose tissue on the arrhythmogenic substrate of AF patients, even without elevated BMI.
- Weight loss through lifestyle changes or surgery is an important pillar in the management of AF in obese patients.