The right ventricle (RV) apex has been the preferred site for ventricle lead placement since transvenous lead implantation for permanent pacing became available half a century ago.1 The RV apex is easily accessible for implantation and yields fixation that is stable in the long term and low capture thresholds. However, there are significant downsides to this approach. Pacing the RV apex results in a non-physiological dyssynchronous ventricular activation, frequently reducing left ventricular (LV) function in the long run.2,3 This so-called pacing-induced cardiomyopathy is associated with an increased risk of AF, heart failure and cardiovascular death.4,5
In a search to prevent pacing-induced cardiomyopathy, alternative pacing sites that maintain interventricular and intraventricular synchrony have been studied intensively. Among the alternative pacing sites, biventricular pacing (BVP), His bundle pacing (HBP) and, more recently, LV septal pacing (LVSP) and left bundle branch pacing (LBBP) have been introduced. This review focuses on the physiology and practicality of LVSP.
Adverse Clinical Effects of Right Ventricular Pacing
The fact that the different ventricular activation sequences induced by artificial electrical stimulation (pacing) influence cardiac pump function was recognised by Wiggers et al. in 1925.6
A subsequent study evaluated the effects of different sequences of ventricular activation on cardiac function in a canine model of complete heart block. This study demonstrated that the haemodynamically more effective LV pacemaker sites resulted in higher cardiac outputs than any of the RV sites tested.7 The differences in cardiac performance between various ventricular pacemaker sites are best explained by varying degrees of dyssynchrony during ventricular contraction.
These findings were later confirmed in multiple animal studies, which showed that the abnormal electrical activation induced by ventricular pacing leads to a depression of systolic and diastolic LV function. The cause of this depression during abnormal electrical activation appears to be a combination of the non-physiological sequence of activation.3
The adverse effects of RV pacing in patients first became apparent in the MOST study, a randomised trial comparing DDDR with VVIR pacing in patients who requiring ventricular pacing because of bradycardia. In this study, in 1,339 patients with a narrow QRS and preserved left ventricular ejection fraction at baseline, it was shown that a high percentage of ventricular pacing was a strong predictor of heart failure (HF) hospitalisations and AF occurrence.5
Later, the DAVID trial demonstrated that, in patients with an ICD indication and reduced LV ejection fraction but without an indication for cardiac pacing, dual-chamber pacing (DDD-70) offered no clinical advantage over ventricular backup pacing (VVI-40). It was shown that dual-chamber pacing was even worse than ventricular back-up pacing, with an increased combined endpoint of death or hospitalisation for heart failure.8 Programming devices to dual-chamber pacing resulted in a ventricular pacing percentage of nearly 60%, while this was only 1% with ventricular back-up pacing.
Physiological Ventricular Activation Sequence
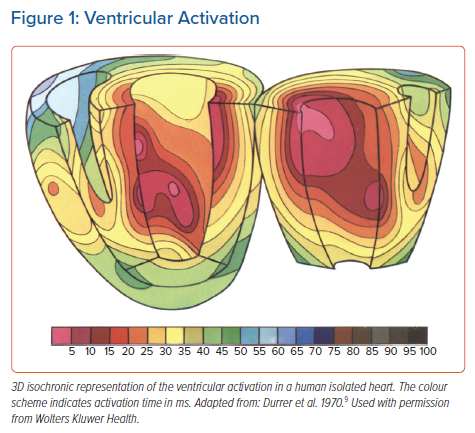
Under physiological circumstances, left ventricular (LV) activation is started from the left bundle branch from three endocardial areas (Figure 1): an area high on the anterior paraseptal wall just below the attachment of the mitral valve; a central area on the left surface of the interventricular septum (IVS); and the posterior paraseptal area at about one-third of the distance from apex to base. In the IVS, activation proceeds from left to right and in an apical-basal direction.9 Although these results were found in perfused isolated hearts, they were confirmed in canine hearts in situ. The pattern of ventricular excitation, as judged from isochrone maps of sections of the hearts, did not change after isolation.9
In addition, it was demonstrated that the activation wavefront spreads much faster around the endocardium than towards the epicardium.9 In other words, endocardial conduction was found to be much faster than endocardium-to-epicardium spread of depolarisation.
Later, more detailed analysis of the spread of activation wavefronts and the distribution of stimulus potentials after epicardial stimulation and endocardial stimulation was performed.10–12 These studies confirmed earlier findings of preferential current flow and more rapid conduction velocity along the myocardial fibre orientation. Subsequent studies showed that myocardial fibres rotate between the epicardial and endocardial surfaces, while most of the endocardial surface contains a layer of Purkinje tissue electrically continuous with the myocardium.13,14 This is potentially one of the main reasons why endocardial spread of activation is faster than endocardium-to-epicardium spread.
Left Ventricular Endocardial Pacing
The sequence of ventricular activation depends strongly on the pacing site. Back in 1988, a study on transmural activation showed there was a close correlation between fibre orientation and the spread of activation within the same plane for (sub)endocardial, midmyocardial and (sub)epicardial stimulation. In addition, conduction velocities were faster for endocardial than for midmyocardial and epicardial stimulation.15 Moreover, activation wavefront spread and conduction velocity during endocardial pacing were found to be similar to that during sinus rhythm.15
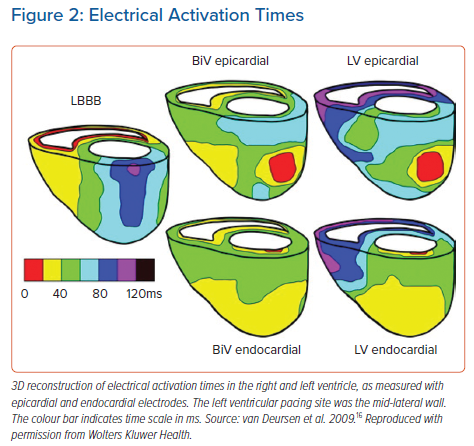
Later, it was also shown that endocardial pacing increased the benefits of cardiac resynchronisation therapy (CRT) compared to epicardial pacing in a canine model of acute left bundle branch block (LBBB).16 Epicardial and endocardial mapping revealed that endocardial BVP reduced the total activation time more than epicardial BVP. Endocardial LV-only pacing resulted in fairly synchronous LV activation whereas, during epicardial LV pacing, large differences in electrical activation times occurred (Figure 2). As a result, three possible mechanisms to explain the more rapid electric activation during endocardial pacing were proposed: a shorter path length of conduction; endocardial conduction is faster than epicardial conduction; and conduction is faster from the endocardium to the epicardium than vice versa.
Preclinical Studies on Left Ventricular Septal Pacing
In a study on ventricular activation and contraction patterns during ventricular pacing, it was demonstrated that, unlike pacing any site in the RV, pacing the left side of the IVS (LV septal pacing) resulted in findings similar to those seen in normal sinus rhythm: the IVS was activated from left to right, the LV pressure rise preceded the right ventricular pressure rise, and there was a normal IVS contraction pattern.17
A subsequent study found that cardiac function in terms of LV stroke volume and LV dP/dtmax was better during LVSP than RV pacing.18 The fact that LVSP maintained cardiac function at a level comparable to normal ventricular activation during sinus rhythm while QRS duration was prolonged confirmed the earlier hypothesis that LV function is dependent on the sequence of activation and not only on the duration of electrical activation. This was later confirmed in animal studies with extensive epicardial mapping and pace protocols. In these studies, multi-LV pacing considerably reduced LV activation time (compared to single-LV pacing), but the improvement in contractility by multi-LV pacing was limited to conditions where single-LV pacing provided only suboptimal improvement.19,20
The activation sequence leading to the best LV pump function is that occurring during sinus rhythm with normal ventricular activation via the His-Purkinje system. Under these physiological circumstances, the electrical impulse exits the Purkinje system at sites on the LV endocardial surface of the septum. It was therefore hypothesised that pacing near LV exit sites of the Purkinje system would result in the most physiological activation and near-normal LV function. An LV pressure-volume analysis of the comparison between RV pacing and various sites within the LV showed, indeed, that LV function was maintained during LVSP when compared to normal ventricular activation, even though activation duration was longer (wide QRS).21 This finding again suggests that a good sequence of electrical activation is sufficient to allow for good LV function.
As a considerable part of the total dyssynchrony in LBBB hearts originate from the delay in conduction across the interventricular septum, a possible role for LVSP was also explored in cardiac resynchronisation therapy. In both ischaemic and non-ischaemic LBBB hearts, LVSP significantly increased LV function compared to baseline LBBB.22
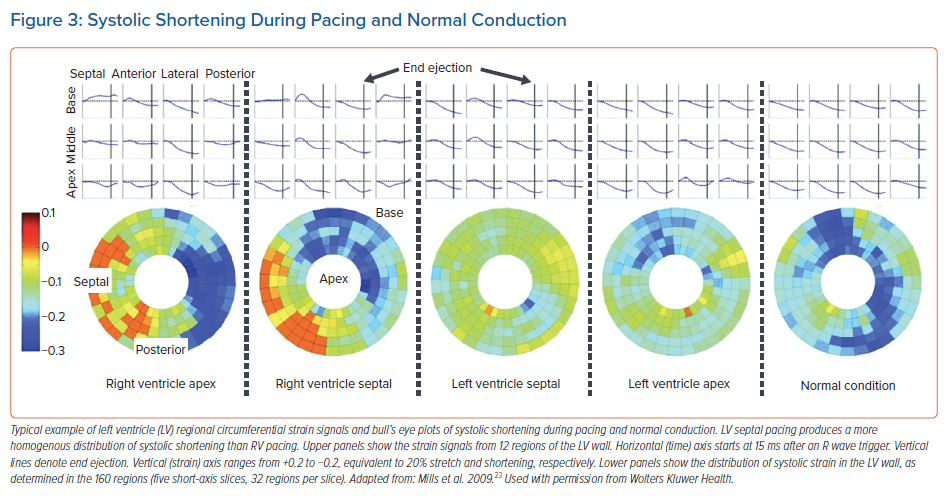
After the acute beneficial haemodynamic effects of LVSP had been shown, the chronic effects of LVSP were studied in canine hearts after 4 months of pacing.23 Again, it was demonstrated that LVSP led to a rapid activation around the LV endocardium, resulting in a pattern that, of all tested pacing sites, most closely resembled the pattern during normal ventricular activation, although RV activation was somewhat delayed. MRI tagging measurements were performed to evaluate myocardial strains, work and indices of global mechanical discoordination (internal stretch fraction) and dyssynchrony (time to peak shortening). The contraction pattern was similar in normal ventricular activation and during LVSP. The pattern of regional time to peak shortening (earliest peak shortening was always observed in the lateral region and shortly thereafter observed in the other three quadrants) was identical for LVSP and normal activation. In addition, the myocardial oxygen consumption and perfusion were determined; LVSP did not significantly alter regional perfusion nor the distribution of regional myocardial work.23 Altogether, MRI tagging analysis showed that LVSP resulted in a homogenous distribution of systolic shortening in time, space and amplitude (Figure 3).
Besides the synchrony of ventricular contraction, temporal changes in haemodynamics and the efficiency of LVSP were studied. Shortly after onset of pacing as well as after 16 weeks, LV contractility and relaxation were comparable between LVSP and normal activation and LVSP maintained native interventricular dyssynchrony.23
Left Ventricular Septal Pacing in Patients
After preclinical studies demonstrated the long-term stability of the LVSP lead, the feasibility of permanently implanting an LV septal lead using the transvenous approach needed to beevaluated in patients.24 The acute haemodynamic effects of LVSP were also studied. In patients with structurally normal hearts with mainly a pacing indication because of sick sinus syndrome, it was demonstrated that LVSP maintained LVdP/dtmax to levels comparable to baseline atrial pacing.24 Importantly, the acute haemodynamic benefit of LVSP over RV apex and RV septal pacing was consistently observed in all patients. Right ventricular septal pacing (RVSP) resulted in a QRS duration of 165 ± 17 ms, while this was 144 ± 20 ms during LVSP. The large difference in QRS duration and haemodynamic effect between RVSP and LVSP, although these sites were only ~1 cm apart, has been related to a significant delay in transseptal conduction during RVSP, which causes considerably later LV mechanical activation and delayed contraction of the LV lateral wall, thereby inducing both inter- and intraventricular dyssynchrony.25
As a beneficial haemodynamic effect of LVSP was demonstrated in canine LBBB hearts, LVSP was studied as an alternative to CRT in patients. An acute haemodynamic pacing study comparing LVSP with BVP was performed in 12 patients with heart failure and an indication for CRT. The acute haemodynamic effect in these patients in terms of LVdP/dtmax was comparable between LVSP and BVP.22
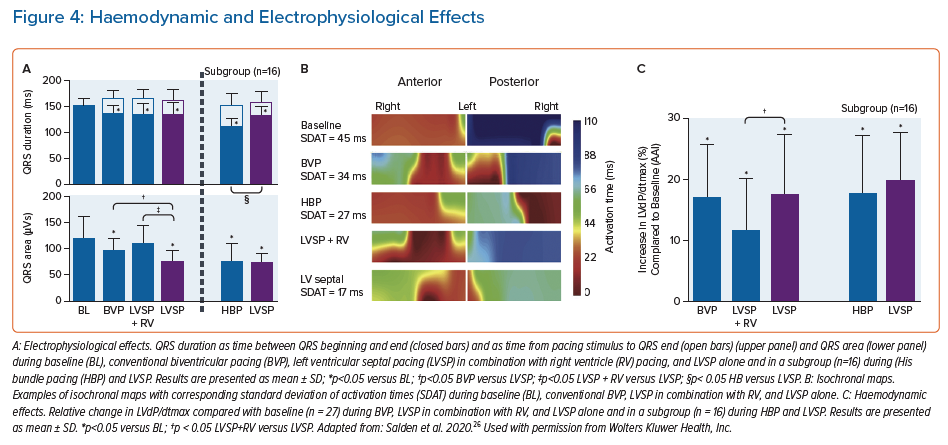
More recently, a more extensive electrophysiological and haemodynamic study was performed in which the acute effects of LVSP were compared with BVP and HBP in patients receiving CRT.26 LVSP was performed using an electrophysiology catheter that was temporarily positioned, retrogradely through the aorta, on the left side of the interventricular septum. The study showed that QRS duration was reduced similarly by LVSP and BVP, and was shortened even further by HBP. In contrast to BVP, LVSP and LBBP, HBP does not produce (additional) ventricular dyssynchrony and QRS duration is therefore shortest during HBP. Although the native His-Purkinje system is engaged in LBBP, QRS duration is similar in LVSP and LBBP. This is because both LVSP and LBBP, while restoring LV activation, induce delayed RV activation, of which the haemodynamic and long-term effects are unknown and need to be carefully evaluated in future studies.26 Interestingly, both QRS area and standard deviation of activation times SDAT – two measures of ventricular dyssynchrony – were significantly smaller during LVSP than BVP, and were comparable to HBP (Figure 4). Also, the increase in LV function (~18%) in these CRT patients, determined by invasive LV dP/dtmax measurements, was comparable for BVP and LVSP as well as HBP.26
Another interesting observation in the study was that no significant differences were found in the electrophysiological and haemodynamic effects of LVSP at the basal, mid- and apical LV septum levels.26 This finding supports the hypothesis that pacing the LV at the endocardium results in fast endocardial spread of activation, probably not necessarily through Purkinje fibres, providing a rather physiological LV activation.
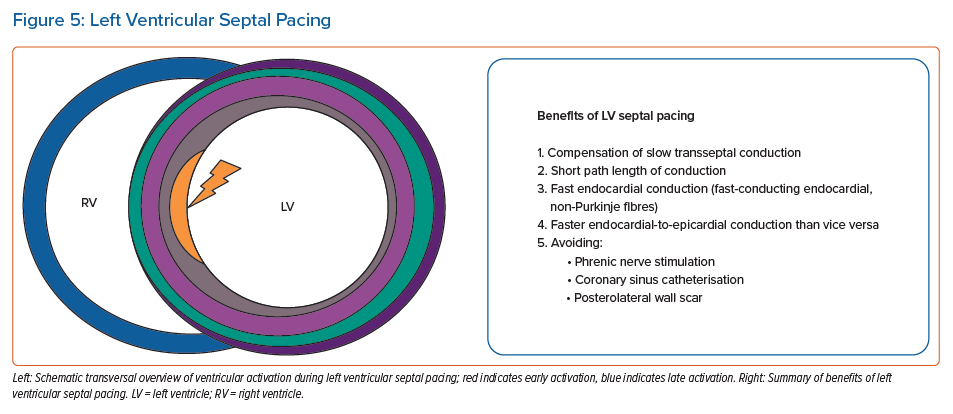
LVSP has been demonstrated to have beneficial haemodynamic and electrophysiological effects in both animal and patient studies (Figure 5). LVSP provides LV synchrony, based on: a shorter path length of conduction compared to epicardial pacing; a faster endocardial than epicardial conduction; and a faster conduction from endocardium to epicardium than vice versa. Furthermore, LVSP avoids the coronary sinus, phrenic nerve stimulation and posterolateral scar in CRT patients.
Practicality of Left Ventricular Septal Pacing
Initial animal studies investigating LVSP were performed using plunge electrodes, where the LV septal endocardium was reached by puncturing the RV free wall and subsequently the interventricular septum.21, 22 Later, clinical studies investigating the acute electrophysiological and haemodynamic effects of LVSP were performed using a steerable electrophysiology catheter advanced retrogradely through the aorta into the LV.22,26
To investigate electric activation and the mechanics, the haemodynamic performance and the efficiency of long-term LVSP, a customised pacing lead with an extended helix was used.23, 24 This Medtronic 09066 lead, a modified 3830 lead with longer, 4 mm screw, was introduced transvenously and, after being positioned against the RV septum using a preshaped guiding catheter (C315His, Medtronic), driven through the IVS until the LV endocardium was reached. After this type of investigational lead had been implanted in animal studies with stable lead measurements for more than 4 months, it was implanted in 10 patients with sinus node dysfunction, using the same transvenous approach, with the lead positioned deep into the IVS.24
In this first-in-human study of long-term LVSP, a 7 Fr preshaped guiding catheter (Model C315-S10, Medtronic) was used, since in HBP its specific shape allowing the catheter tip to be positioned perpendicularly against the IVS. Then, the implanter, using RAO and LAO views, positioned the tip of the lead to the middle of the IVS, guided by an RV angiogram. In the initial procedures, intracardiac echocardiography was used to verify the position of the lead tip on the IVS before screwing the lead into the IVS.
Positioning the lead mid-ventricular in the IVS is different from the more recently applied left bundle branch pacing (LBBP), where the lead is placed closer to the anatomical level of the His bundle. Importantly, capture of the left conduction system was not studied nor pursued in this research. While rotating the lead, the implanter repeatedly assessed the IVS penetration depth by injecting small amounts of contrast medium through the guiding catheter against the IVS under fluoroscopy in LAO.
In addition, pacing was repeatedly performed from the tip electrode while advancing the helix through the IVS to assess changes in paced QRS morphology that indicated that the left side of the IVS had been reached, i.e. a right bundle branch block-like QRS morphology. Pacing thresholds and impedances were measured to ensure that the helix did not protrude in the LV cavity. Images showing the implantation process are shown in Figure 6.
Practical Limitations of Left Ventricular Septal Pacing
Although the initial studies on long-term LVSP were performed with a modified version of the Medtronic 3830 lead (with an extended helix), it was recently shown that penetration of the septum was possible using the standard Medtronic 3830 lead, which is the most frequently used lead nowadays.27
Despite the straightforward procedure, there are factors that possible complicate implantation. LVSP lead implantation failure is likely to result from difficulty in lead fixation, which is usually caused by septal hypertrophy and/or scar/fibrosis. In addition, tissue lodging into the helix induced by the drill effect and insufficient sheath support or reach is an issue.
Advances in dedicated implantation tools may overcome some of these practical issues. Structural evaluation of the heart, especially the interventricular septum thickness and the presence of septal scarring, can be beneficial. In the presence of septal hypertrophy or scar, reaching the far subendocardium, where the left bundle branch is situated, can be challenging. In these cases, LVSP can be of particular benefit since the more complicated targeting of the His-Purkinje system is not required. A recently published study investigating ventricular synchrony during transventricular pacing demonstrated that deep LVSP produces LV synchrony comparable to LBBP.28
Left Ventricular Septal Pacing Versus Left Bundle Branch Pacing
Left bundle branch area pacing (LBBAP; LVSP and LBBP) has recently been introduced as alternative method of conduction system pacing to maintain left ventricular synchrony.27 Multiple studies have demonstrated the safety and feasibility of LBBAP. However, reported left bundle branch capture rates differ, but are usually between 60% and 90%.28–30 Consequently, up to one-third of patients who are reported to have been treated with LBBP were in fact treated with LVSP.
Unlike LVSP, LBBP and HBP engage the intrinsic His–Purkinje system and LBBP has been shown to maintain ventricular synchrony at levels comparable to HBP and even to intrinsic ventricular activation.31–35 Non-selective HBP, however, is claimed by some to be less beneficial. A possible explanation could be capture of only the right bundle branch, since published data suggest benefits to patients receiving selective or non-selective HBP, and ultra-high frequency ECG analysis has demonstrated that both types of His bundle capture preserve ventricular electrical synchrony.36 Capture of the right bundle branch without the left bundle branch results in delayed LV activation. In (especially selective) LBBP, there is delayed RV activation but this is likely to have fewer clinical implications (RBBB patients versus LBBB patients). In contrast, LVSP results in direct left-to-right septal activation and interventricular dyssynchrony is less with LVSP than with LBBP.37
A recently published study comparing LBBAP with and without evidence of direct capture of the left conduction system showed that LV dyssynchrony is comparable between LVSP and LBBP.28 This study showed that, compared to RV pacing, QRS area and left ventricular activation time (now referred to as V6RWPT) decrease when the lead is advanced towards the LV subendocardium. A reasonably acceptable level of ventricular dyssynchrony is achieved when an R’ (right bundle branch block-like QRS morphology) becomes apparent in lead V1. The R’ in lead V1 indicates delayed RV activation and therefore suggests that the left part of the IVS is reached. A significantly lower QRS area during LBBP compared to LVSP was found, although the absolute difference was small.
Another recent study compared differences in ventricular depolarisation between LVSP and LBBP using ultra-high-frequency ECG (UHF-ECG).37 UHF-ECG analysis showed that, although during LBBP left ventricular lateral wall activation is faster compared to LVSP, LBBP results in greater interventricular dyssynchrony. Interventricular dyssynchrony is less in LVSP than in LBBP, since left-to-right transseptal depolarisation occurs immediately after pacing and left subendocardial Purkinje fibres are captured later, which results in a more balanced ventricular depolarisation.37
Developments in the Transseptal Approach
As with all new techniques, implantation of the ventricular lead via the transseptal approach is subject to development. In the initial patient study that demonstrated the feasibility of LVSP, total procedure time decreased from 237 minutes in the first patient to 83 minutes in the last. RV angiography and intracardiac echocardiography was performed to verify the position of the lead tip on the IVS before screwing the lead into the IVS.24 The experiences gained during this study taught us that, with the pre-shaped guiding catheters, leads were always directed towards the septum and that the additional RV angiogram and intracardiac echocardiogram are not necessary, which facilitates the procedure.
The method as described by Huang et al. to perform permanent LBBP and evaluate left bundle branch capture is rather complex as it requires relatively advanced electrophysiological knowledge and electrophysiological equipment in the cath lab.38 The technique calls for simultaneously recording the 12-lead ECG and the intracardiac electrogram from the lead tip for the assessment of paced QRS morphology and measurement of intervals, while carefully advancing the lead transseptally. In addition, the His bundle potential is searched for as reference point and the left bundle branch potential needs to be recorded. Furthermore, multiple repeated measurements are required to diagnose capture of the left bundle branch.39
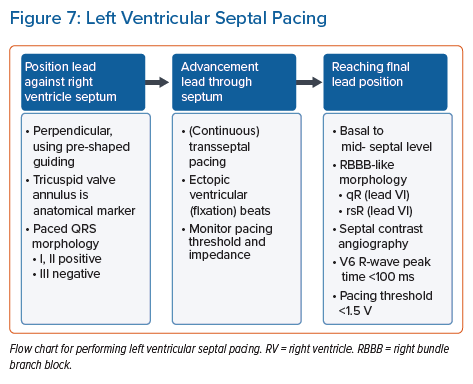
In contrast, the LVSP implantation procedure is fairly straightforward to perform as the specialised His-Purkinje system is not targeted. As the exact septal position of the lead in LVSP is less critical compared to LBBP, there is no need for the recording of a His bundle or left bundle branch potential. After the lead has be positioned perpendicularly against the septum, the lead is fixated somewhere in the basal to mid-level of the septum. The tricuspid valve annulus, visible on standard fluoroscopy imaging, is used as an anatomical marker.
The paced QRS morphology, visible on standard 12-lead ECG recording, is used to determine whether the initial position on the RV septum is valid. Preferably, a QRS morphology with a positive QRS complex in leads I and II and a negative QRS complex in lead III are seen while pacing the RV septum. Advancement of the lead through the septum is guided by QRS morphology, especially in lead V1, either via continuous pacing or by evaluating ectopic ventricular beats occurring during lead rotations for deep intraseptal deployment (fixation beats).40–41
The suitability of the final lead position can be determined from the standard 12-lead ECG, where preferably a paced ‘qR’ morphology in lead V1 is seen, indicating the lead is deployed deep within the left septum. Besides the right bundle branch block-like QRS morphology induced by left-sided septal pacing, deep septal deployment can also be confirmed by septal contrast angiography. QRS duration during LVSP is comparable to BVP and LBBP, but prolonged compared to HBP.22,26,28,37
A flow chart for performing LVSP is shown in Figure 7.
Conclusion
The severity of impairment of ventricular function induced by pacing depends largely on the site of pacing. The conventional and frequently used RV apex is a reliable and easy to reach position, but – in a subset of patients – using this site can increase cardiac morbidity and mortality.
Recently investigated techniques of pacing, such as LVSP and LBBP, seek to avoid pacing-induced cardiomyopathy leading to this. LVSP provides ventricular synchrony, based on compensation of slow transseptal conduction, short path length of conduction and fast endocardial conduction, and has been demonstrated to result in acute electrocardiographic and vectorcardiographic results comparable to BVP.
LVSP can be a valuable alternative to LBBP, especially in patients where capture of the left bundle branch is impeded. The long-term clinical effects of LVSP and LBBP and the differences between them are still unknown and need to be investigated.
Clinical Perspective
- Left ventricular septal pacing(LVSP) and left bundle branch pacing (LBBP) have been introduced to maintain or correct (left) ventricular (dys)synchrony. While LBBP produces better intra left ventricular synchrony, LVSP provides better interventricular synchrony, based on compensation of slow transseptal conduction, short path length of conduction and fast endocardial conduction.
- LVSP has been demonstrated to result, in the short term, in electrocardiographic and haemodynamic results comparable to biventricular pacing.
- The LVSP procedure is more straightforward than that for LBBP. LVSP can be a valuable alternative to LBBP, in particular in patients where capture of the left bundle branch is impeded.
- The long-term clinical effects of LVSP and LBBP and the differences between them are still unknown and need to be investigated.