In the context of ischaemic heart disease, ventricular tachycardia (VT) occurs due to reentry over what are classically thought to be fixed anatomical structures. The most common cause of a structural barrier is scar tissue, and remodelling over time within the scar results in regions of patchy and poorly coupled recovered fibres that serve as surviving electrical channels for slow conduction.1–3 These channels within the scar contain regions of slow conduction and conduction block, creating an environment in which protected isthmuses can sustain VT.1,2 Reentrant circuits most commonly involve VT traversing a circuitous, and often zigzag course through these surviving fibres before exiting from a stable location to activate the entire myocardium, before returning in diastole into the scar region.1,4,5 A single substrate region may facilitate multiple VT circuits, via multiple channels and exits, and may evolve over time to facilitate new circuits due to abnormal remodelling, resulting in recurrent VT. Treatment with anti-arrhythmic drugs, which predominantly act to delay recovery from excitation or slow conduction, often fails to prevent reentry, and large-scale studies confirm their poor efficacy.6,7 Thus catheter ablation of VT remains the mainstay of treatment for patients with VT when conservative measures fail. This review summarises current VT ablation techniques as well as emerging data on functional assessment of substrate characteristics.
Conventional Ablation Techniques and Outcomes
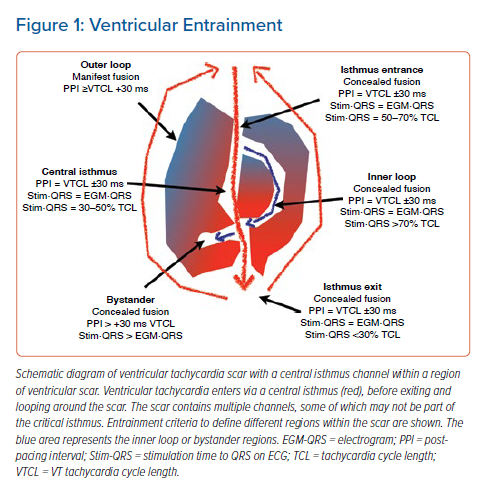
Entrainment Mapping
Early catheter mapping studies were able to identify that complex signals with decremental properties are present within the diastolic pathway of the tachycardia.8 This led to the development of entrainment mapping techniques (Figure 1) pioneered by Stevenson et al.4,9 The process involves applying stimuli within regions of myocardium thought to be part of the VT circuit, and assessing the timing of the return cycle. Entrainment mapping of VT remains the gold standard for identifying critical sites for ablation of VT, but this technique remains a challenge in patients in whom VT is poorly tolerated or non-sustained.4,9 Additionally, the process can be time consuming, requiring serial roving of the catheter to critical regions, pacing manoeuvres and measurement of time intervals. The challenges and limitations of this methodology have been previously reviewed.10 Commonly this technique is performed with a 3.5 mm tip ablation catheter that contains a relatively widely spaced bipole, increasing the susceptibility to far-field signals or far-field capture. Such pitfalls of entrainment are well-documented.11
Activation Mapping
Activation mapping, similar to entrainment mapping of VT, relies on mapping in VT and carries similar limitations. It involves the collection of the timing points of the majority of the VT circuit, which then enables the construction of a colour map of activation, and visual classification of the critical isthmus. New high-density mapping systems may allow complete characterisation of VT circuits in suitable patients, suggesting that VT circuits may be more complex than the standard entrainment models described, with regions of slow conduction at the entrances and exits of VT isthmuses.9,12
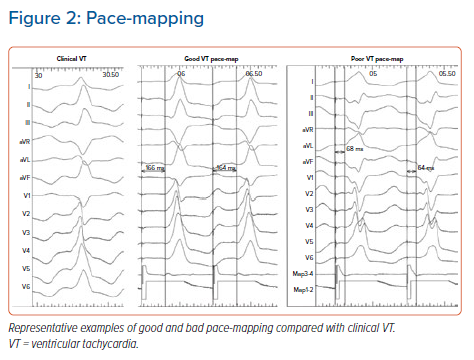
Pace-mapping
In patients in whom it is not possible to perform entrainment mapping, pace-mapping may be a strategy to identify the critical isthmus.11,13,14 This strategy involves pacing within regions of the heart, to capture regions near the critical isthmus myocardium that result in wavefront exit to the same region as the clinically documented VT, with subsequent matching of the 12-lead ECG (Figure 2). Again, this method has its limitations, including the fact that it is time consuming, that pacing rate may influence QRS morphology due to conduction changes within critical regions, that paced QRS morphology can vary over a narrow range of areas, and that pacing output and catheter size can significantly affect the region of tissue captured, resulting in bystander capture.15 Additionally, the technique is designed to identify the exit site of a reentry circuit, which may not always be the optimal site for ablation.
Substrate Mapping
In light of the limitations of the above methods, several substrate mapping and ablation methods have been developed.16,17 The goal of these strategies is to target the abnormal tissue that sustains VT. This is particularly useful when VT is non-sustained or poorly tolerated. Strategies include linear ablation within scar tissue, scar homogenisation, scar dechanneling, core isolation, ablation of late potentials (LPs) and ablation of local abnormal ventricular activity (LAVA).16,18–22 Again all have limitations, including that the definition of scar is variable (dependent on mapping technology and on the technology used to define scar such as MRI), and that the definition of abnormal potentials may be subjective.23,24 LPs are defined on electrograms as double or multiple components separated by an isoelectric or very-low-amplitude interval >50 ms.21 LAVA is defined as sharp high-frequency ventricular potentials, of low amplitude, distinct from the far-field ventricular electrogram occurring any time during or after the far-field ventricular electrogram in sinus rhythm (SR), displaying fractionation or double or multiple components separated by very-low-amplitude signals or an isoelectric interval.22 Specifically, LAVA lateness is affected by the location they are mapped to in the heart. LAVA mapping may miss critical arrhythmogenic substrate, in the septum and other early-to-activate regions.25 Additionally, the mapping catheter used can significantly affect the substrate characterisation, with multipolar catheters favouring the delineation of higher definition near-field components.12 Another limitation is that LAVA and LPs may play a bystander or an active role in the VT circuit, and there is no clear way to differentiate between these regions. The use of new high-density mapping catheters may help to identify important electrogram features such as LPs with low amplitude and short duration, which may better delineate the VT isthmus.26
Outcomes
Outcomes from VT ablation vary, with an average freedom from appropriate ICD shock of 72% for ablation versus 60% for medical therapy in the major randomised trials.27 This highlights the fact that current ablation strategies do not decrease the mortality rates, although reduced hospitalisation, improved quality of life and greater cost-effectiveness have been noted with VT ablation.28,29 Additionally, real world outcomes may be worse than in the controlled environments of many trials: outcomes as poor as 44%, major complication rates of up to 12%, a 3.5% rate of cardiac tamponade and a rate of death of up to 2.7% at 30 days have been reported.30 Thus, there is a need for improvements in real world ablation strategies, both to improve the outcomes and safety of the procedure, and to offer patients a better standard of care. Non-inducibility at the end of ablation has been shown to be a strong predictor of outcome, but this is not always achieved in clinical practice.31,32
Functional Substrate Mapping
Requirement for Functional Substrate Assessment
Classical substrate mapping techniques predominantly involve mapping ventricular scar substrate in intrinsic rhythm, however, VT circuits may be dynamic, and substrate characteristics may not be static or prevalent in intrinsic rhythm. Interrogation of VT initiation from device tracings suggests that VT is frequently triggered by extrasystolic impulses that alter the conduction and refractory properties of the tissue to enable initiation of VT.33,34 This suggests that dynamic substrate changes may unmask critical conduction changes that facilitate functional unidirectional block and reentry, as has been demonstrated in animal and computer models.35,36 In the light of this, functional substrate mapping techniques have been developed, to unmask critical substrate changes that may play a part in VT mapping.
Existing Function Substrate Mapping Techniques
Decrement-evoked Potential Mapping
Decrement-evoked potential (DEEP) mapping utilises drive train pacing at 600 ms (S1) from the right ventricle (RV), followed by the delivery of a single extrastimulus (S2).37,38 The process involves looking at the behaviour of LPs in response to this S1–S2 pacing protocol. If the difference between the time interval measured from surface ventricular far-field signal onset to the local LP bipolar electrogram during the S1 drive, and the same interval measured immediately after the S2 is >10 ms, the LP is defined as a DEEP. The same strategy is used for multicomponent electrograms from which DEEP are identified if their components split by >10 ms after S2. DEEP LPs were co-localised with the regions of the initiation and diastolic circuit of VT more accurately than those areas displaying non-decremental LPs. At 6-month follow up 75% of patients were free of any VT, after ablation to DEEP regions plus further ablation if VT was still inducible.37 This highlights the potential for targeted functional substrate mapping, looking specifically for functional decrement in LP, which may be the precursor to unidirectional block and VT initiation. Although promising, repeated stimuli may be time consuming, and DEEP software is not commercially available, meaning that manual measurement annotation and tagging of regions would be required at present to replicate this technique. This may prolong procedure time, and repeated drive train pacing may risk worsening of heart failure in a cohort of patients who are already unwell with poor ejection fraction (EF), therefore larger scale trials are awaited.
Evoked Delayed Potential Mapping
Evoked delayed potential (EDP) mapping similarly uses RV pacing to invoke functional masked substrate changes.39 Electrograms are compared during SR, RV pacing at 500 ms, and short coupled single S1–S2 stimuli. This technique uses a 3.5-mm-tip mapping catheter placed in a stable position. Electrograms are systematically analysed during SR, RV pacing at a fixed rate of 500 ms, and during the application of a single RV extrastimulus (S1–S2) with a coupling interval of 50 ms above the ventricular refractory period, over the presumed infarct area as derived from imaging data (echocardiogram and contrast-enhanced MRI) regardless of local electrogram amplitude or morphology during SR. Sites are examined manually and those exhibiting low-amplitude (<1.5 mV) near-field potentials with conduction delay >10 ms or block in response to RV extrastimuli are categorised as EDPs and annotated on the map. Substrate modification aimed at EDP elimination is then performed. LPs (onset after QRS complex, separated by an isoelectric segment from the far-field signal >20 ms) during SR or RV pacing without additional conduction delay during RV extrastimuli are not targeted. In an initial study of patients undergoing this procedure compared with a historical cohort of patients matched for left ventricular function and electroanatomical scar area, patients in the hidden substrate group had a higher 1-year VT-free survival (89% versus 73%), suggesting that functional substrate mapping may improve outcomes compared with standard protocols.39 However, again this protocol requires accurate manual electrogram annotation/analysis, which may be time consuming, especially with the use of multi-electrode mapping catheters.
Isochronal Late Activation Mapping
Isochronal late activation mapping involves mapping in SR or intrinsic pacing if the patient is pacing dependent.40,41 Although not strictly a dynamic form of mapping, it does endeavour to delineate functional properties within the tissue. Each abnormal electrogram is manually annotated to the offset of the local bipolar electrogram deflection in realtime, signifying the completion of local activation to incorporate the latest local activation into the map.
Isochronal crowding is analysed relative to the entire ventricular activation window, and candidate deceleration zones (DZs) are defined as regions with >3 isochrones in a 1 cm radius. Extreme conduction slowing is defined as regions of isochronal crowding with continuous local fractionated activity within the DZ. Electrograms within candidate DZs are manually confirmed to have discontinuous fractionated characteristics or split local activation. Discontinuous electrograms require verification by the operator in real time with the concordant local timing at adjacent sites within 1 cm. Abnormal electrograms without reproducibility and confirmatory neighbouring electrograms are deleted. At 12 months, 70% freedom from VT recurrence (80% in ischaemic cardiomyopathy and 63% in non-ischaemic cardiomyopathy) was achieved.40
Sense Protocol Mapping
Practical Considerations
Dynamic changes in conduction and repolarisation within this substrate may form a critical aspect of the tachycardia mechanism when conduction velocity slows dynamically and tissue refractory periods lengthen. We have previously demonstrated dynamic conduction and repolarisation changes within myocardial scar and regions of LP.42 In light of this, we developed a short coupled single extrastimuli protocol (sense protocol; SP) to evoke maladapted conduction delay within the tissue and map these critical regions of slow conduction and hidden abnormal electrograms.43 One of the limitations of the DEEP and EDP mapping methods is the need for multiple pacing, sometimes from multiple sites, which can be time consuming, involve extensive manual annotation and measurement of electrograms, and which risks putting patients into heart failure or poorly tolerated VT through repetitive stimulation. Repetitive stimulation may also cause conduction block during pacing, which may result in failure to see critical slow conduction regions that may play a crucial role in the tachycardia mechanism.22,43 Additionally, interrogation of VT initiation from device tracings suggests that VT is frequently triggered by single extrasystolic impulses, and therefore we developed the SP to replicate the physiological substrate properties of VT initiation.33,34,43 This enables a consistent, easily reproducible functional substrate mapping technique with rapid acquisition of dynamic substrate maps.
Mapping Technique
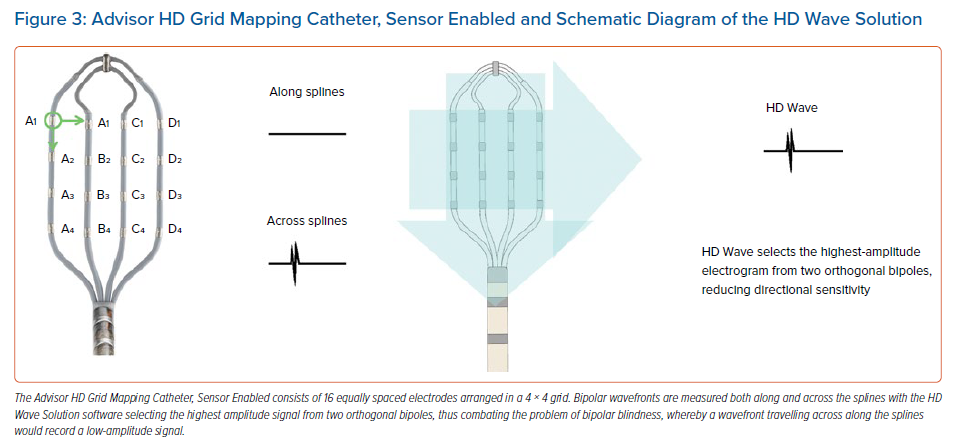
VT substrate maps are acquired with the EnSite Precision mapping system (Abbott) and the Advisor HD Grid Mapping Catheter, Sensor Enabled (Abbott; Figure 3), which is a multipolar mapping catheter containing 16 equally spaced electrodes in a 4 × 4 grid layout (Figure 3). A hexapolar catheter is placed in the RV apex for pacing, with the proximal pole located in the inferior vena cava blood pool to reference for unipolar signals. In view of the finding that VT is frequently triggered by extrasystolic impulses, substrate maps of bipolar voltage and LPs were obtained simultaneously during:
- intrinsic or SR; and
- the paced beat of a single (without drive train) sensed extrastimulus from the RV apex (SP) at 20 ms above the effective refractory period, which is applied every fifth beat, in order to allow the conduction properties of the tissue to return to steady state.33,34
Bipolar LP substrate maps are collected using the HD Wave Solution mapping technology of the Advisor HD Grid Mapping Catheter, Sensor Enabled (Abbott), whereby bipolar recording along and across splines is enabled, with the system analysing orthogonal bipolar wavefronts and recording the highest amplitude of the two signals to negate the effect of wavefront directionality. The system is set to annotate the latest LPs identified within the diastolic window. Additionally, the system uses the Best Duplicate algorithm, which analyses orthogonal bipoles, collecting multiple electrograms beat by beat for a specific point on the map, in order to compare signal amplitude for the collected mapping data; and then automatically selects the electrogram with the highest peak-to-peak voltage in a collected region to display on the map. These features negate the effects of sampling quality, beat-to-beat changes and wavefront in relation to the bipolar orientation on the recorded electrogram. A new window of interest is set in the mapping system that contains the entire diastolic interval, and the TurboMap feature is used to identify the latest LPs from SP pacing. This enables simultaneous mapping of SR and SP to speed up the mapping process.
For the purposes of scar/voltage delineation, normal myocardium is defined as tissue with a bipolar voltage >1.5 mV, dense scar was defined as a bipolar voltage <0.5 mV, and scar border zone was defined as a bipolar voltage 0.5–1.5 mV.
Examples From Sense Protocol Mapping
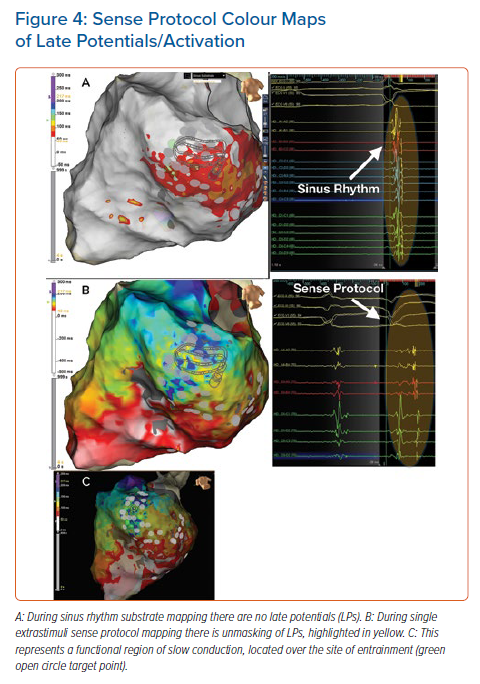
The main principle of functional substrate mapping is to expose hidden substrate and electrogram changes that are functional and which, by nature of their decrement/unmasking, may play an important role in the critical regions of slow conduction within the ventricular scar that facilitate VT. Figure 4 shows an example of a region in which there were no LPs in SR (Figure 4A), but LP were unmasked through SP mapping (Figure 4B); this region correlated with the region of best entrainment on VT mapping (Figure 4C).
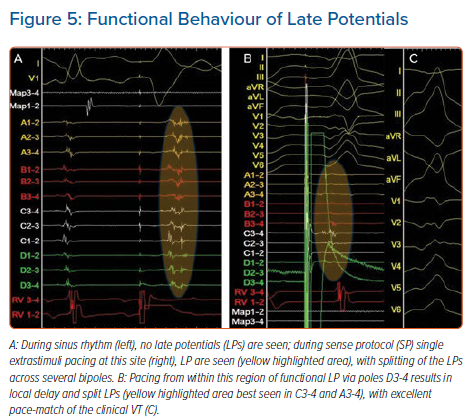
Additionally, in response to the dynamic stress of the SP on the conduction properties, electrograms not only display delay/unmasking, but also display complex splitting of components. Figure 5A shows an example of unmasking of LP during SP mapping, with electrogram splitting and delay, with long fractionated components between them (see splines A1-2 in Figure 5A). These are markers of functional slow conduction tissue and regional conduction block in response to SP stimuli that are not seen during SR mapping, and pace-mapping from within this region (Figures 5B and 5C) resulted in a good match for the clinical VT (Figure 5C).
Outcomes of Functional Substrate Mapping
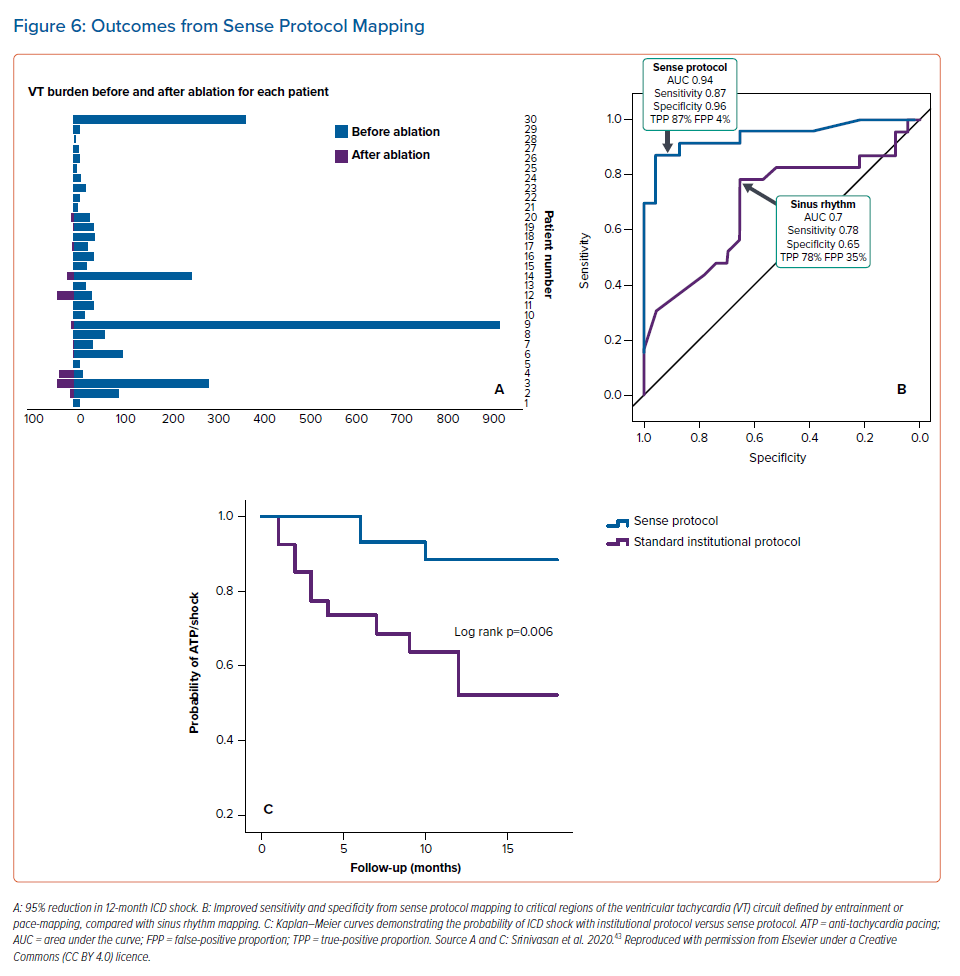
When compared with a case-matched institutional cohort (for age, sex and EF), outcomes from SP VT ablation showed a 90% freedom from VT, compared with 60% in a historical institutional cohort, using conventional mapping methods.43 VT burden was reduced by 95% (Figure 6A).43 This is similar to the 75% freedom from VT at 6 months seen with DEEP mapping and the 89% 1-year VT-free survival of EDP mapping compared with 73% in a historical matched cohort.37,39 This may be related to the increased sensitivity and specificity of SP mapping to critical regions of the VT circuit (Figure 6B). SP mapping resulted in a lower probability of ICD shock compared with the standard institutional VT ablation protocol (Figure 6C).43 If regions that display functional changes and delay, dynamically relate to critical circuits within scar and scar border zone in ischaemic VT, this may prove to be an important step in improving success in VT ablation, by minimising the risk of mapping in VT and offering improved functional targets for substrate-guided ablation.
Conclusion
Despite the improvement in catheter mapping technologies over the last 20 years, long-term outcomes of VT ablation continue to remain poor in real world data.30 Activation and entrainment mapping of VT is limited in patients in whom VT is not inducible or not tolerated, and puts patients at risk of decompensation in terms of cardiac and renal function due to the neurohormonal consequences of VT. Additionally, activation/entrainment mapping fails to account for the fact that there may be several channels of tissue capable of sustaining VT, and limiting mapping to induced VTs fails to account for the potential for other regions of diseased tissue to sustain further VT in future. Better understanding of the critical substrate that may facilitate VT is needed. This may be achieved through the use of newer high-density mapping techniques that enable clearer characterisation of the substrate, and through new novel techniques of assessing dynamic substrate changes, aided by the advances in computing power of modern mapping systems.26,43,44 The majority of centres worldwide perform substrate mapping in a static state either during SR or paced rhythm. As a consequence of this, mortality improvements from VT ablation have not been demonstrated in large-scale randomised trials incorporating standard mapping techniques.45 This may be a function of a failure to significantly improve outcomes compared with medical strategies. Emerging data suggest that substrate changes are not static, and that mapping dynamic changes may improve outcomes.43 Larger scale randomised comparator trials are required to investigate whether functional substrate mapping techniques may improve long-term outcomes and mortality in VT ablation. Additionally, further studies of these techniques in non-ischaemic, septal, intramural and epicardial circuits are required.
Clinical Perspective
- Mapping ventricular tachycardia (VT) remains a challenge due to the difficulties associated with haemodynamic stability and sustaining VT to enable conventional mapping and entrainment.
- Outcomes from conventional substrate mapping techniques remain poor in real world data.
- VT circuits are known to be dynamic, and emerging functional substrate mapping techniques suggest that unmasking or delay in local electrograms may represent surrogate markers for regions of conduction delay that are critical to the VT circuit.
- Further large-scale randomised studies of VT ablation comparing functional substrate mapping techniques with conventional techniques are required.