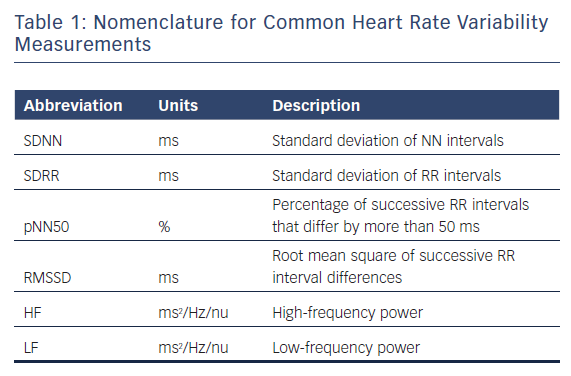
Estimation of Prognosis Using HRV
The association of heart rate variability (HRV) and prognosis, both for all-cause and cardiovascular (CV) mortality, has been studied using ECG at rest, with exercise and in the ambulatory setting. A meta-analysis by Hillebrand and colleagues found that, using both resting and ambulatory ECG monitoring, lower HRV is associated with a 32–45 % increased risk of first CV event in patients without known CV disease.1 Additionally, elevated HRV demonstrates a protective effect, with an increase in standard deviation of the normalised NN interval (SDNN) of 1 % resulting in an approximate 1 % reduction of fatal or non-fatal CV disease event.
Resting ECG for HRV Prognosis
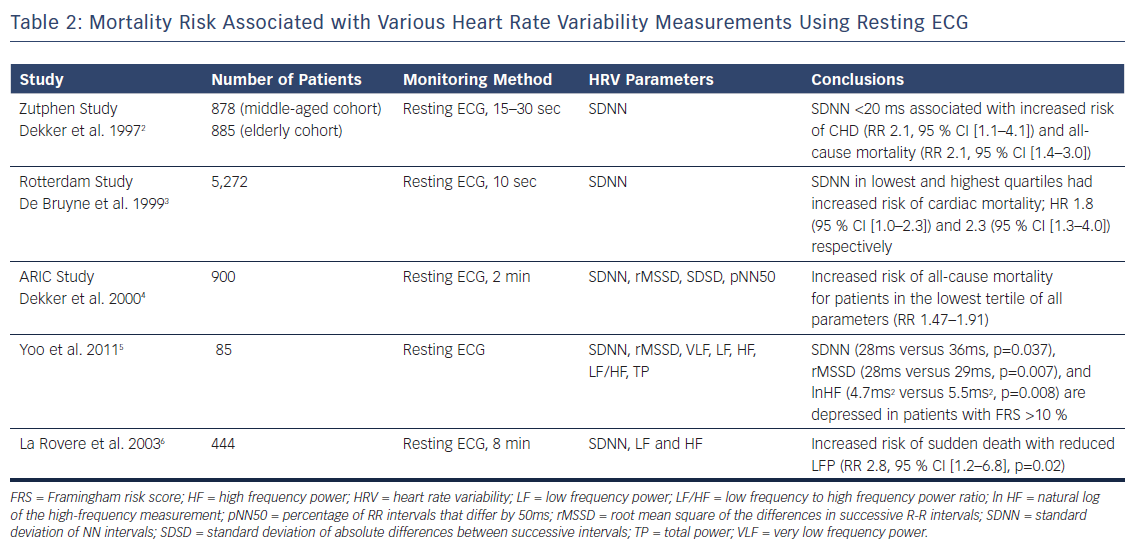
The Zutphen study assessed HRV obtained from resting ECGs in 878 men aged 50–65 years, referred to as the middle-aged cohort, who were followed up 15 years later.2 Participants from the original population and new patients formed a group of 885 patients, referred to as the elderly cohort. Using resting 15–30 seconds of 12-lead ECG data to calculate SDNN, the investigators found increased rates of coronary heart disease mortality (RR 2.1, 95 % CI [1.1–4.1]) and all-cause mortality (RR 2.1, 95 % CI [1.4–3.0]) among patients with HRV <20 ms (compared with 21–39 ms) in the middle-aged cohort at 5-year follow-up. No significant change in mortality was noted in patients with the highest HRV (≥40 ms). No association with mortality was found in the elderly cohort.
The Rotterdam study enrolled 5,272 patients aged 55 years (mean = 69 ± 9) and acquired 10-second rest 12-lead ECGs.3 SDNN values were put into quartiles with the 25th, 50th, and 75th percentiles corresponding to values of 9.6 ms, 15.2 ms, and 25.9 ms, respectively. The investigators found that patients in the lowest quartile had an 80 % increase (HR 1.80, 95 % CI [1.0–3.2]) for cardiac mortality compared with patients in the third quartile after adjustments for age and sex. Patients in the highest quartile had the most pronounced adjusted risk for cardiac mortality (HR 2.3, 1.3–4.0), suggesting that low or high SDNN can be associated with mortality in an older population.
The Atherosclerosis Risk In Communities (ARIC) study used a case-cohort method of analysing 900 patients without CAD and using 2-minute ECGs, SDNN, root mean square of the differences in successive R-R intervals (RMSSD), and percentage of R-R intervals that differ by 50 ms (pNN50).4 Demographic-adjusted survival analysis showed increased RR of all-cause death and incident coronary artery disease in the lowest tertile compared with intermediate and highest tertiles for all variables. RR of mortality for SDNN in the lowest tertile (<30 ms) was 2.10 compared with the intermediate group.
Yoo and colleagues compared HRV with the Framingham Risk Score to determine if HRV values could serve as an acceptable substitute for a CHD risk assessment.5 The study involved 85 adults using resting ECG measurements in the seated position taken after 20 minutes of rest. Patients with Framingham risk >10 % were found to have significantly depressed SDNN, RMSSD, and in high-frequency (HF) values. These signals, however, appeared to be isolated to men and there was no statistically significant relationship between Framingham risk score and HRV among women in this small cohort.
These findings extend to cohorts with established CV disease. Studying only patients with dilated cardiomyopathy, La Rovere and colleagues found reductions in HRV to be predictive of sudden death.6 A resting ECG of 8 minutes’ duration was obtained with spontaneous respiration, as well as a controlled breathing period. Patients with dilated cardiomyopathy were found to have reductions in low frequency (LF) power with spontaneous (30 ms2 versus 45 ms2, p=0.01) and controlled-breathing (28 ms2 versus 41 ms2, p=0.02). Reduced LF power during controlled breathing was found to be predictive of sudden death during a 3-year follow-up period (RR 2.8, 95 % CI [1.2–6.8]), independent of left ventricular dimensions.
Resting HRV measurements have been used to link enhanced sympathetic autonomic nervous system (SANS) activity with myocardial destabilisation and arrhythmogenic potential. HRV, however, serves as an indirect measurement of SANS, given its action on baroreceptors and the sinoatrial node. Periodic repolarisation dynamics (PRD), which can be obtained by focusing on low-frequency patterns of resting 3D ECGs, may serve as a more direct measurement.7 Factors that lead to heterogeneous sympathetic activation, such as previous MI, diabetes, and inherited channelopathies, are all associated with greater PRD at rest. Previous studies have shown resting PRD to predict post-MI mortality independent of established risk factors such as left ventricular ejection fraction, the Global Registry of Acute Coronary Events (GRACE) score, increased QT-variability index, and reduced HRV.8 Current studies are ongoing to better understand the role of PRD in identifying patients for prophylactic ICD placement.
Ambulatory ECG for HRV Prognosis
While the ECG is the established gold standard for cardiac monitoring, technological advances have allowed for commercial products to be developed to allow for out-of-hospital monitoring. Both ECG and photo-based plethysmography have been validated as accurate measures of HR, and commercial products using these methods of detection have allowed for accurate and prolonged detection of arrhythmias such as AF.9 With increasing evidence for HRV as a prognostic tool, there have been attempts to validate these home-based technologies for HRV as well.
Studying patients from the original Framingham study, Tsuji et al found an increased risk of all-cause mortality in patients with depressed HRV.10 Using 2-hour ambulatory ECG monitoring, the investigators considered 736 patients from the original Framingham study. They found that, with adjustment for age, sex, and clinical risk factors, patients 1 SD below the mean for SD of NN intervals (SDNN), LF, very LF (VLF), HF and total power all had increased risks of all-cause mortality over 4 years. SDNN was the only time-domain factor with elevated risk after adjustment (HR 1.38, 95 % CI [1.13–1.70], p=0.019). Decreased values of LF were associated with highest adjusted risk (1.70, 95 % CI [1.37–2.09], p=0.001) of all HRV parameters. Additionally, a study combining patients from the original Framingham study and the Framingham Offspring Study (n=2,501, average age 53 years), demonstrated that with 2-hour ambulatory ECG monitoring, patients with lower SDNN had significantly higher rates of heart disease (12.40 versus 2.06).11
A long-term prospective study by Kikuya reported results from ambulatory BP data obtained from 1,542 subjects (mean 62 years, 40 % male) in Japan.12 BP and HR measurements were taken every 30 minutes using an ambulatory cuff-oscillometric device, as the patients went about their normal day and night routines. Patients were divided by SDNN into three groups (mean - SD, mean ± SD, mean + SD) for analysis. A significant inverse linear relationship was noted for daytime HRV and cardiovascular death (p=0.008) during the mean follow-up period of 8.5 years, even after adjustment for use of beta blockers. The lowest HRV tertile was found to have HR of 3.64 (p=0.02). Changes in night-time HRV did not show a similar risk of CV death.
The Autonomic Tone and Reflexes After MI (ATRAMI) study considered the prognostic value of SDNN in 1,284 patients with recent MI.13 Patients with MI within the previous 28 days were enrolled in the study and followed with ambulatory Holter monitoring for SDNN analysis. Baroreflex sensitivity was also assessed by measuring rate-pressure response to. infusion of phenylephrine. Multivariate analysis showed that patients with SDNN <70 ms or baroreflex sensitivity <3 ms/mmHg showed increased risk of cardiac mortality. In patients with depression of both parameters, 2-year mortality was 17 % compared with 2 % in patients where the parameters had remained stable. Klieger et al. demonstrated similar post-MI results,with a RR of mortality of 5.3 in post-MI patients with SDNN <50ms compared with those that had >100 ms.14 The Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI-2) study analysed the usefulness of HRV in patients who had a recent MI that had been treated with fibrinolytic therapy.15 Of 567 patients, 7.8 % died of cardiovascular causes during 1,000 days of follow-up. HRV parameters exhibited elevated RR for SDNN (RR 3.0), RMSSD (RR 2.8) and NN50 (RR 3.5).
While often used to predict mortality, a review by Reed et al looked at HRV as a predictor of ventricular tachyarrhythmias (VTA).16 Vybrial et al showed no consistent changes in HRV indices in 24 patients who wore Holter monitors and developed ventricular fibrillation.17 Huikuri et al, however, found significant reductions in HR and HRV indices (SDANN, HF, LF) in post-MI patients who developed ventricular tachycardia compared with those without arrhythmic events.18 These changes occurred in the 1-hour period preceding VTA, and the degree of reduction was more pronounced in patients with sustained arrhythmias. Shusterman et al demonstrated that the presence of a change in HRV alone,19 within a 2-hour period before arrhythmic events, could predict the development of VTAs.20
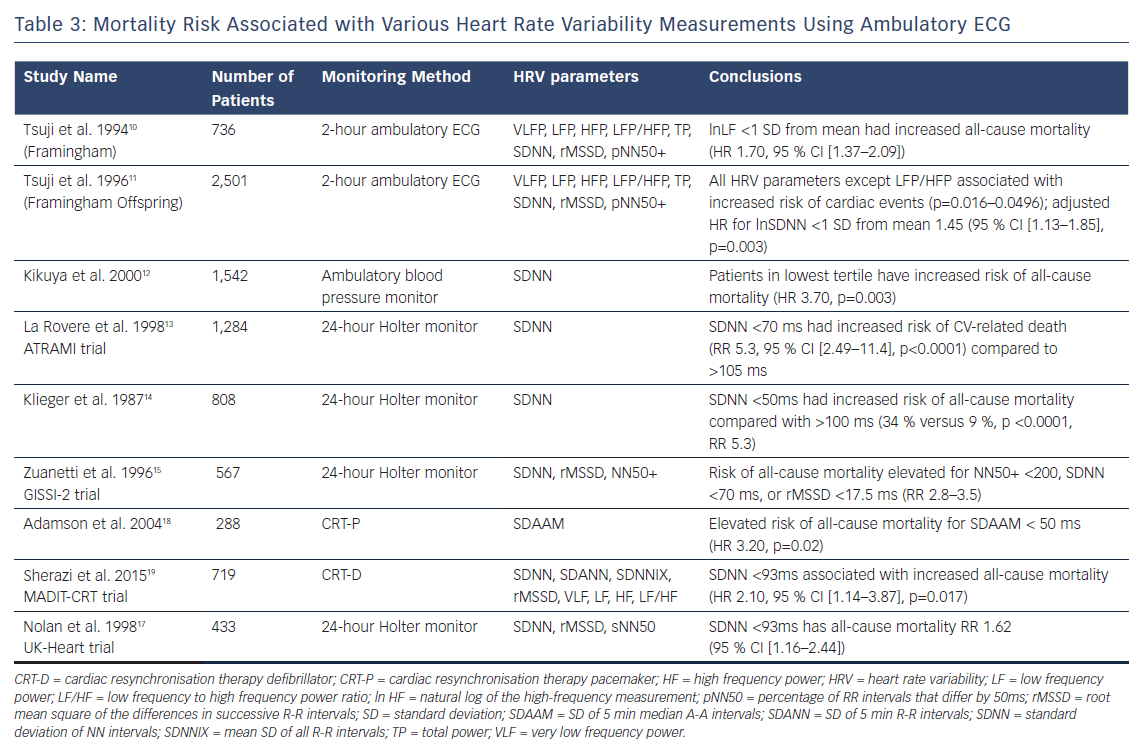
Focusing on patients with an ejection fraction lower than 35 %, New York Heart Association (NYHA) functional class III or IV, and QRS duration >130 ms, Adamson and colleagues sought to assess the feasibility of implantable cardiac resynchronisation devices for prognostic purposes in an ambulatory setting.21 Using atrial intervals, HRV was defined as the standard deviation of median 5-minute a-a intervals over each 24-hour period (SDAAM). They found that the patients with the lowest SDAAM (<50 ms) had the highest all-cause mortality (HR 3.2, p=0.02) and CV-related death (HR 4.4, p=0.01) compared with higher SDAAM values over a 12-month follow-up period. Additionally, absolute SDAAM values were lower in the inpatients who were included in the study.
The Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) trial followed patients with ejection fraction lower than 30 %, QRS duration >130 ms, and non-ischaemic heart failure with NYHA functional class I or II, randomising them to either cardiac resynchronisation therapy (CRT-D) or ICD alone.22 In a retrospective analysis of these patients, Sharazi et al found that patients in the lowest tertile of SDNN (≤93 ms) had higher rates of death or heart failure (24 % versus 17 %, p=0.004).22 Similar outcomes were shown using frequency domain measures such as VLF (28 % versus 14 %, p<0.001). The overall results agreed with the UK Heart Failure Evaluation and Assessment of Risk Trial (UK-Heart) trial, which showed increased risk of death in patients with heart failure and depressed SDNN values.20 In their sub-study analysis, the MADIT-CRT investigators concluded that ambulatory HRV analysis in heart failure patients may identify patients who would most benefit from CRT; low HRV showed no benefit with CRT-D versus ICD alone, while patients with preserved HRV treated with CRT-D had a lower risk of death. It appears that not only can HRV be used to assess the risk of death and hospitalisation in patients with heart failure, but it may also be used to determine candidates for CRT-D therapy.
Exercise HRV and Prognosis
In the first study of exercise, HRV and prognosis, Dewey and colleagues performed time and frequency-domain HRV analysis on R-R interval data taken from 1,335 subjects (95 % male; mean age 58 years) during the first and last 2 minutes of treadmill testing and the first 2 minutes of recovery.23 Cox survival analysis was performed for the 53 cardiovascular and 133 all-cause deaths that accrued during the 5-year mean follow-up. After adjusting for potential confounders, greater root mean square successive difference in R-R interval during peak exercise and recovery, greater HF power and percentage of HF power, lower percentage of LF power, and lower ratio of LF to HF during recovery were significantly associated with increased risks for all-cause and CV death. Of all time-domain variables considered, the log of the root mean square successive difference during recovery was the strongest predictor of CV mortality (adjusted HR 5.0, 95 % CI [1.5–17.0]) for the top quintile compared with the lowest quintile). Log HF power during recovery was the strongest predictor of CV mortality in the frequency domain (adjusted HR 5.9, 95 % CI [1.3–25.8], for the top quintile compared with the lowest quintile). They concluded that exercise-induced HRV variables during and after clinical exercise testing strongly predict both CV and all-cause mortality independent of clinical factors and exercise responses in a clinical population.
Despite the strong association found in the study by Dewey et al., other investigators, such as Nieminen et al., have argued that these findings may be predominantly driven by heart rate alone.23,24 As HRV is associated with HR physiologically (autonomic system) and mathematically (R-R interval), further consideration of this relationship is required to integrate these variables in risk stratification. Pradhapan and colleagues explored this by assessing the effect of HR correction on pre- and post-exercise HRV.25 They selected 1,288 patients from the Finnish Cardiovascular Study cohort. Inclusion criteria included completing a maximum effort exercise test and good quality HRV measurement for at least 2 minutes during rest, immediately before exercise and during post-exercise recovery. All participants were followed for cardiac and non-cardiac mortality for a mean time of 54 months. The investigators concluded that exercise-induced HRV parameters (RMSSD, VLF power, LF power, p<0.001 pre- and post-exercise) strongly predict cardiac morality with similar but weaker association found for non-cardiac mortality. Consistent with contemporary data presented by Sacha et al, they showed that when predicting both cardiac and non-cardiac morality, weakening HRV dependence on HR at rest improved prognostic capacity.26 Future studies are required to quantify the clinical significance of HRV recorded during exercise with a different HR and respiratory rate.
Rest and Exercise HRV for Training
The use of HRV as a tool to track and monitor the status of athletes has gained much interest over recent years.27 The desire to be the best often pushes the athlete to the fine line between the maximisation of effective training (achieved by duration, frequency and intensity) and ineffective training (e.g. maladaptation, non-functional overreaching and overtraining).28 Given the fact that adaptive responses to a training load or stimulus are individual,29–31 it is understandable that the ability to independently assess positive or negative training adaptation would be advantageous to athletes, sport scientists and coaches alike.
HRV and Training Maladaptation
The hypothesis behind the early detection of non-functional overreaching (NFOR) or overtraining (OT) and fatigue is the possibility of assuring adequate recovery through specified rest between training. By allowing recovery based on the constantly changing dynamic of the athlete and the amount of further training needed, the recovery optimises future performance. The performance begins to decline if the recovery is not adequate, resulting in a continuum from functional overreaching (F-OR), NFOR, OT and, eventually, overtraining syndrome (OTS).32
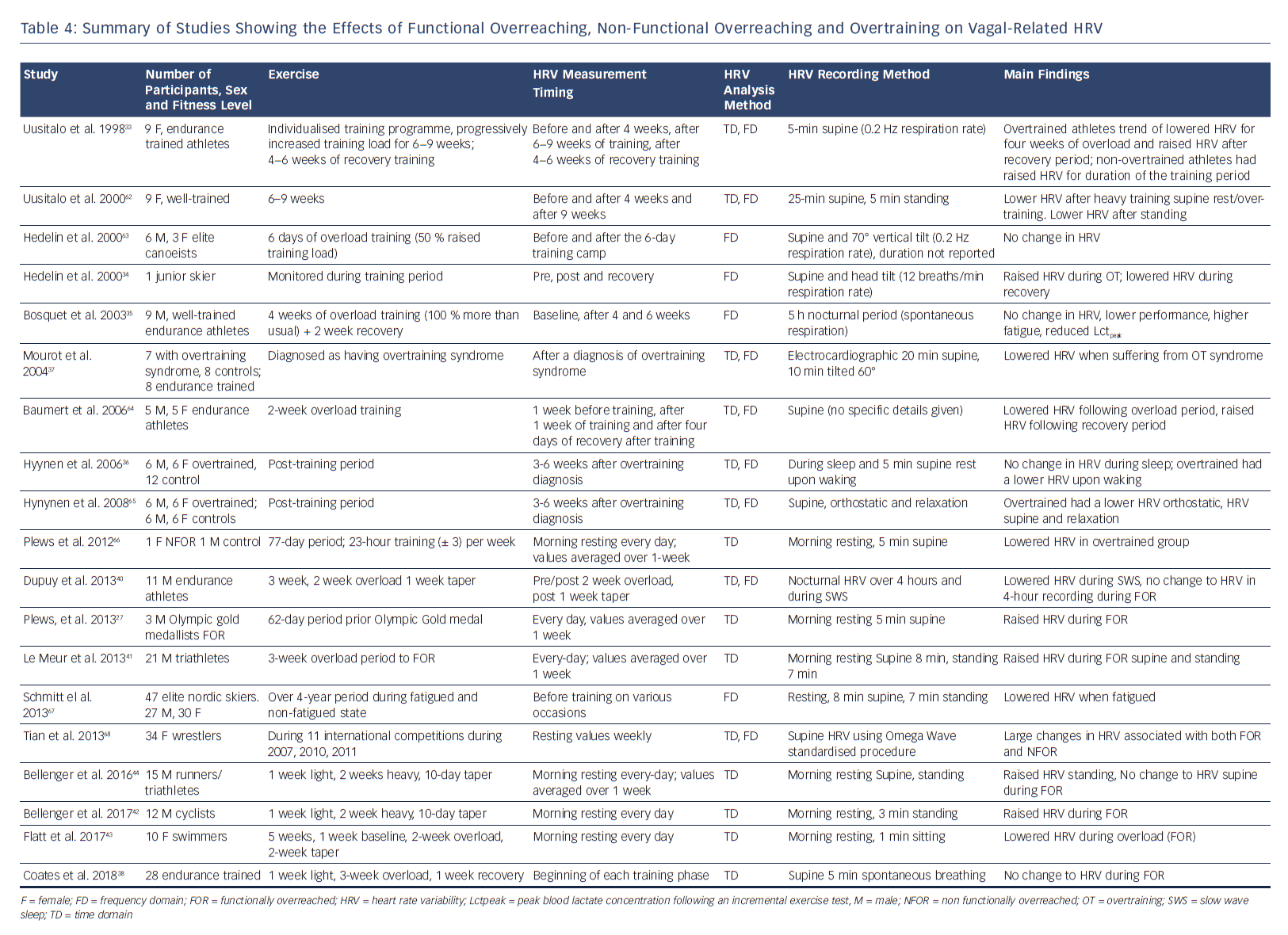
In athletes, changes in the patterns of their autonomic nervous system (ANS) reflected by altered HRV may serve as useful objective parameters for managing their physical fatigue. Information regarding the extent to which the body recovers after training may provide useful data to avoid NFOR, OT and OTS. Many studies have examined HRV and overtraining have revealed ambivalent findings, with increases, decreases, and no change in HRV reported (Table 4).33–36 In one case study, a junior skier with reported OT had a substantially increased HRV and the values subsequently decreases once the athlete had undergone a recovery period.34 Conversely, Mourot et al.37 showed that overtraining was associated with decreased HRV. Seven athletes had endurance training and had been clinically diagnosed with OTS. However, given the continuum from F-OR to OTS, and the difficulty in deciphering between stages, these differences observed may be due to inconsistencies in the accurate diagnosis of the fatigue stage.38,39 This may be one of the reasons why more recent studies have focused on F-OR rather than NFOR and OT,which can be quantified by decreases and subsequent increases in performance (after a taper period).38,40–42 Accordingly, these data demonstrate the importance of understanding where each athlete sits on this continuum of fatigue (F-OR→NFOR→OT→OTS). Such knowledge is critical for the accurate interpretation of HRV results to regulate athlete training.
Plews et al. showed substantial reductions in HRV in an NFOR elite triathlete before a competitive race.27 It was suggested that the equivocal findings in HRV studies considering NFOR, OT or OTS, may also be due to problems with recording methodologies. As day-to-day HRV values are too variable, the authors demonstrated that when HRV were averaged over a 1-week period, they consistently showed substantially lower HRV values because of NFOR. Such findings have been subsequently supported by other research studies.41,44,45
HRV and Training Adaptation
Endurance training elicits marked changes in cardiorespiratory function in both sedentary and active individuals, concomitant to changes in cardiac vagal activity, as evidenced by reduced resting and exercise HR.46 As such, the individualised nature of changes in HRV is fundamental to its use as a marker of training adaptation.47
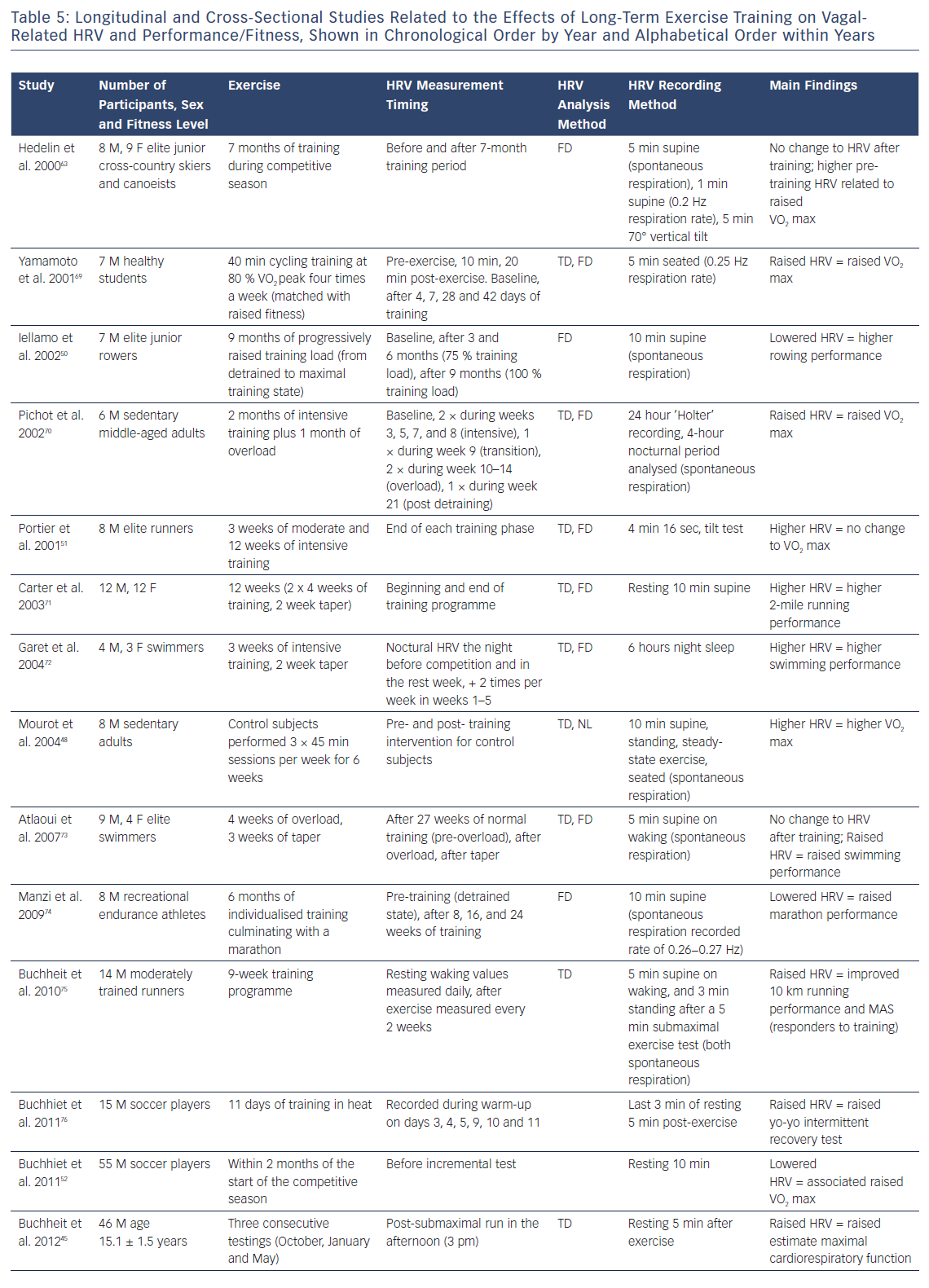
The changes in HRV in response to endurance training programmes have been extensively studied (Table 5). In people who have been sedentary or who have trained recreationally, endurance training for 2, 6 and 9 weeks has been shown to induce parallel increases in aerobic fitness and HRV.45,48,49 For example, previously sedentary men completed 9 weeks of intensive endurance training followed by 4 weeks of overload training and had large increases in maximal aerobic capacity (+20 %) and vagal-related HRV (+67 %).50 While this is the response typically seen in sedentary and recreationally trained people after a period of endurance training, the response in people who have an extensive training history can be decidedly different. In these athletes, the HRV response to training is inconsistent, with longitudinal studies showing no change in fitness (VO2 max uptake) despite increases in HRV, and cross-sectional studies showing lower HRV in association with superior fitness.52,52 In elite distance runners training for 18 weeks (6 weeks moderately intensive and 12 weeks intensive) culminating in a half marathon or marathon, there was no change in VO2 max, but a 45 % increase in HRV.45 Conversely, in 55 young male soccer players, lower HRV was associated with higher VO2 max and maximum aerobic speed.49
Plews et al. used data from elite rowers at the Olympics.53 They showed a consistent HRV trend before peak performance, with substantial increases in HRV (above baseline) before a decline to baseline values as the competition approached (during a taper period).Such trends have since been validated in experimental studies by both Le Meur et al. and Bellenger et al. with athletes functionally adapting to training (F-OR).41,44 Le Meur et al. showed that triathletes who responded positively to 3 weeks of overload training had substantial increases in RMSSD (96 % chance of an HRV increase) followed by reductions to baseline after a 1-week taper. Those classified in the F-OR had large increases in running performance over an incremental running rest (effect size 1.17 ± 0.22). Similarly, Bellenger et al showed HRV increases in triatheletes (effect size = 0.62 ± 0.26) after a 2-week heavy training period. These increases were reduced after a 10-day taper which coincided with improved 5 km running time trial performance (effect size -0.34 ± 0.08). Importantly, in both these studies, there were observed reductions in performance after the training overload period, when HRV was substantial higher. Accordingly, in such cases, increases in HRV are indicative of coping with intensified training (i.e. F-OR), not increases in performance. Improved performance was only observed after the taper period when HRV had reduced back towards baseline levels.
Taking these data into account, it has been suggested that there is a bell-shaped relationship between vagally related HRV and fitness/performance.49 This, to some extent, may also be due to HRV saturation which is often seen in athletes with extensive training histories and low resting heart rates.54
Using HRV to Guide Training
Given the usability of HRV to track training adaptations, it could be used as a tool to guide daily training. Three studies have shown training guided by the daily recordings of HRV to be superior (at increasing fitness and exercise performance) to training based on conventional methods.55–57
Recently, Vesterinen et al. investigated the effectiveness of using HRV to prescribe training on a day-to-day basis.57 Forty recreational endurance runners were divided into the HRV-guided experimental training group (EXP) and traditional pre-defined training (TRAD). After a 4-week preparation training period, the TRAD group trained according to a predefined training programme including two-to-three moderate (MOD) and high-intensity training (HIT) sessions per week during an 8-week intensive training period. The timing of MOD and HIT sessions in EXP was based on HRV measured every morning. RMSSD was used to prescribe training because of its greater reliability than other HRV spectral indices.58 A 7-day rolling average of RMSSD was calculated because it has been proposed to be more sensitive to track changes in the training status compared with single-day values.53 The MOD/HIT session was programmed if HRV was within an individually determined smallest change. Otherwise, low-intensity training was performed. VO2 max and 3,000 m running performance were measured before and after both training periods. The number of MOD and HIT sessions was significantly lower (p=0.021, effect size 0.98) in the EXP group (13.2 ± 6.0 sessions) compared with TRAD (17.7 ± 2.5 sessions). No other differences in training were found between the groups. The 3,000 m run time improved in EXP (2.1 % T 2.0 %, p=0.004) but not in the TRAD group (1.1 % T 2.7 %, p=0.118) during the intensive training period. A small but clear between-group difference (effect size = 0.42) was found in the change in running performance over 3,000 m. VO2 max improved in both groups (EXP: 3.7 %, ± 4.6 %, p=0.027; TRAD: 5.0 % ± 5.2 %, p=0.002). They concluded that there was potential in using resting HRV to prescribe endurance training by individualising the timing of vigorous training sessions.
Two studies by Kiviniemi et al. also showed superior responses to training when guided by daily HRV, similar to Vesterinen et al.55–57 Greater improvements in VO2 max and maximal attainable workload56 in groups of trained subjects who performed high-intensity training when morning resting HRV was high, and low-intensity training when these values were low. This was despite the HRV-guided training group performing HIT sessions less frequently than a traditional training group (average three compared with 4-hour HIT sessions per week). Hence, adaptation was improved when lower intensity training was completed when vagal modulation of HR was attenuated.
A study from 2018 split 17 well-trained cyclists into two groups.59 Group one followed a training plan guided by morning resting HRV (HRV-G, n=9), whereas group 2 followed a more traditional approach (TRAD, n=8). Following a similar design to Kiviniemi, on days when the 7-day rolling average of RMSSD fell outside the predetermined individual smallest range, the HRV-G training group would carry out low intensity training or rest rather than moderate-intensity training or HIT.55 The TRAD group’s training regimens included scheduled low-intensity, moderate-intensity, HIT and high-intensity interval training. There were no statistical differences in volume or intensity distribution in either group during the experimental period. Although there were no between-group differences, the HRV-G group substantially increased in peak power output (5.1 ± 4.5 %; p=0.024), upper threshold power (13.9 ± 8.8 %; p=0.004) and 40 km time trial performance (7.3 ± 4.5 %; p=0.005). The TRAD group did not improve significantly in any of these performance measures after their training period. This again supports the possible efficacy of HRV-G training being a suitable method to enhance training adaptations in athletes.
Methodological considerations are important when using HRV to monitor training in athletes. However, it is generally accepted that reductions in HRV are associated with negative performance outcomes, and increases associated with a positive response to higher training loads (Tables 4 and 5). However, such changes must be taken within the context of the training phase (i.e heavy training versus taper), and fitness status of the individual.60 Both supportive and opposing views have been highlighted in a recent HRV and exercise training meta-analysis by Bellenger et al.61 The aim of this meta-analysis was to interpret how vagally derived indices of HR could be used to inform training decisions. Focusing on HRV only here, they suggested that improved exercise performance was associated with increases in resting RMSSD (effect size = 0.58). However, there was also a small increase in resting RMSSD (effect size = 0.26) associated with decreases in performance. This supports the idea that, although HRV measures can be useful, they should still be used alongside other measures of training tolerance to aid decision-making.
Conclusions
The past decade has shown that HRV provides valuable prognostic information that can contribute to risk scores and cardiac variables such as echocardiographic measurements and exercise capacity. There is strong evidence to suggest that elevated HRV has a protective effect against CV disease. Conversely, exercise and HRV show the opposite relationship. Greater HRV during recovery from exercise is associated with an increased risk of all-cause and cardiovascular death. However, other investigators have argued that these findings may be predominantly driven by heart rate alone and further research is required in this area. Over more recent years, the area of HRV and athlete monitoring has been investigated. It is now generally accepted that substantial reductions in HRV are associated with negative adaptations to training and HRV increases are associated with positive adaptations, with an inverted U shape being the optimal trend in HRV in athletes before they reach peak performance. Furthermore, studies that have based daily training sessions on morning resting HRV values have had mostly positive outcomes.
In the era of wearable monitoring devices and increased interest in personalised approaches to lifestyle modification, HRV may provide useful information to direct lifestyle change, guide exercise regimens and monitor for over-training. Given the advancement in wearable HRV recording devices, research is needed to understand the complex relationships between physiology and performance and day-to-day trends. Furthermore, future population studies are needed to assess the potential of HRV information acquired through wearable devices which use photo-based plethysmography and ECG technology and validate its value as a prognostic marker.
Clinical Perspective
- Resting, exercise and ambulatory heart rate variability (HRV) measurements are useful for predicting cardiovascular risk.
- The use of mHealth technologies allows acceptable ambulatory detection of HRV compared with traditional ECG methods.
- The data behind using ambulatory HRV to guide or structure athletic training programmes are limited, but there is a possible benefit in using HRV to optimise performance and prevent over-training