AF is the most common sustained cardiac arrhythmia and radiofrequency is the dominant energy source used for atrial ablation. Owing to an ageing population and the increasing burden of cardiovascular disease, the prevalence of AF, particularly in developed countries, is increasing.1,2 With increasing prevalence comes additional financial burden.3 It is paramount that AF therapy is effective in reducing morbidity and improving quality of life. Evidence that AF is frequently triggered by ectopy arising from within the pulmonary veins led to the development of pulmonary vein isolation as a widely practised therapy and circumferential pulmonary vein isolation is now fundamental to the vast majority of AF ablation procedures.4–6
Success in AF ablation requires the creation of a contiguous, transmural and electrically isolating ablation scar that surrounds the pulmonary veins while avoiding collateral tissue injury.2,7,8 Radiofrequency ablation lesion formation is dependent on several key variables, including power and current delivery, duration of energy application, catheter contact, orientation and stability.7,9,10 Conventionally, pulmonary vein isolation radiofrequency ablation has employed low-power, long-duration (LPLD) generator settings with the aim of producing mature ablation lesions while minimising complications including steam pops, cardiac tamponade, pulmonary vein stenosis and collateral tissue damage.3,11 Conventional settings at most ablation centres are usually in the region of 25–35 W for 30–60 seconds per lesion.12,13
As an alternative, ablation using high-power, short-duration (HPSD) generator settings was originally suggested in 2006, yet relatively few trials have assessed its performance and comparability to conventional settings.14 Nevertheless, recent simulation studies and small clinical trials have shown potential advantages of high-power short duration ablation as a viable alternative treatment strategy. Definitions of what constitutes high power shows considerable variability within the literature, which suggests this in a range of 50–90 W. While there have been a number of computer simulation and experimental studies delivering power at the upper range, far fewer clinical trials have adopted this approach because of concerns regarding patient safety. For the purpose of this article, high power is defined as the use of an ablation generator power output of 50 W or above.
This article reviews the data on HPSD ablation as an alternative to conventional radiofrequency ablation. After briefly describing the fundamental principles underlying ablation lesion formation, the potential advantages of HPSD ablation are explored. The function of a new ablation catheter aiming to improve the safety of HPSD ablation is described and gaps in knowledge together with suggestions for future research in this area are identified.
Radiofrequency Ablation Lesion Formation
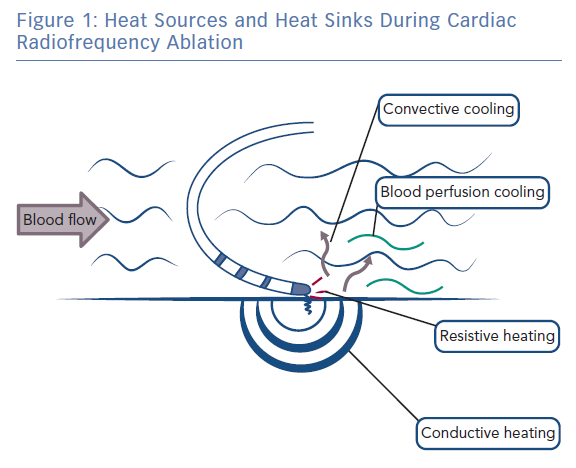
During radiofrequency ablation, electromagnetic energy is converted to thermal energy, resulting in tissue heating and destruction.15 Tissue heating occurs via two mechanisms: resistive (direct) and conductive (indirect) heating (Figure 1). Resistive heating is an active process and generated when the radiofrequency energy applied encounters an impedance at the catheter electrode/myocardium interface and within the myocardium itself. Resistive heating is rapid, beginning and ending immediately with the initiation and cessation of radiofrequency energy application. Conductive heating is a passive process as heat is transferred away from the ablation lesion core. Conductive heating takes anywhere from 30 seconds to 2 minutes to reach a stable state of thermal equilibrium.7,16 If radiofrequency delivery is halted before this, conductive heating will continue after the termination of radiofrequency energy delivery.
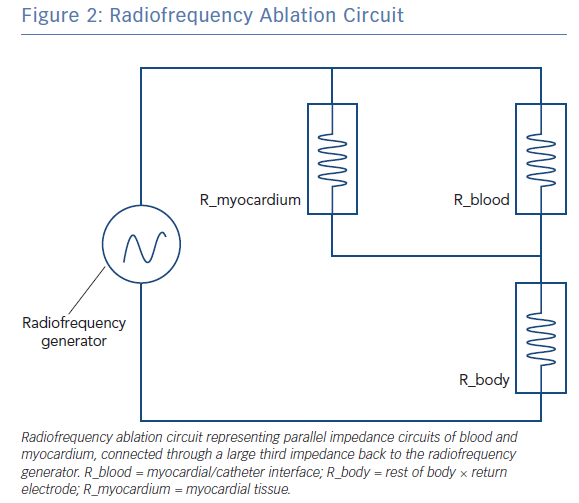
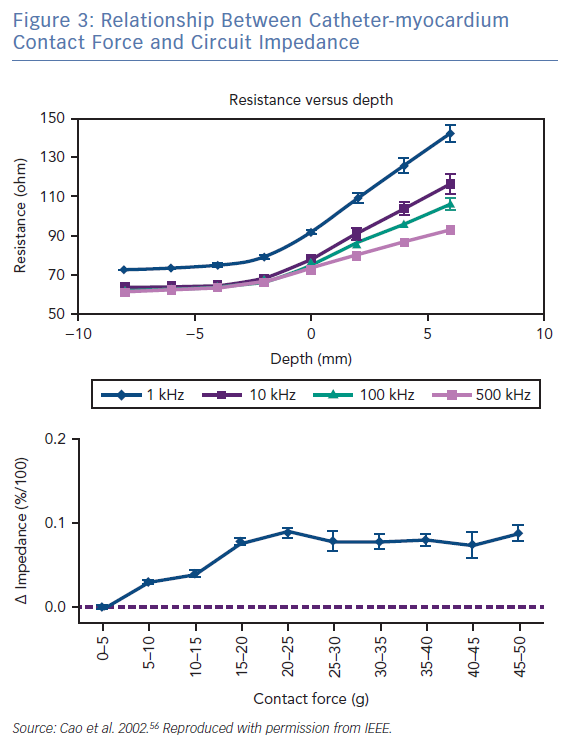
The radiofrequency circuit can be thought of as two impedances in parallel (that of blood and that of myocardium), connected through a large third impedance (the body) back to the radiofrequency generator (Figure 2). Much of the energy delivered during radiofrequency application does not heat myocardium, but is dissipated within blood, which is a superior conductor to tissue due to not only its lower impedance but also increased catheter contact given its liquid state.16 Energy delivery can be further altered by the degree of catheter contact at the tissue interface. Total circuit impedance has been shown to rise in vitro as the catheter is progressively pressed into myocardium; less of the ablation electrode is then in contact with the highly conductive blood pool, increasing Rmyocardium and decreasing Rblood (Figure 3A). Data from the authors’ laboratory has shown these observations reproduced during in vivo atrial ablation (Figure 3B, unpublished data). As the distance between the catheter and the target ablation site increases, energy delivery is reduced by as much as 75%.15 Further energy is absorbed in large nearby structures, such as the left lung, which is wrapped around the left atrial wall and may account for some of the conflicting evidence between in vitro and in vivo findings.14 Subsequent ex vivo and computational modelling studies have attempted to simulate a similar environment to that seen in vivo to recreate a realistic heat dispersion model of a beating heart.17 With general assumptions made regarding catheter contact, typical blood flow and impedance of surrounding structures, only 9% of total energy delivered will contribute to the creation of a standard lesion.16
Key to understanding how this energy delivery results in tissue heating is the distinction between power (measured in watts) and energy (measured in joules). While power describes the rate at which energy is consumed per unit time, it is the quantitative property of energy that must be conferred on an object to perform work on that object. Power and energy are therefore linked by the following formula:
Energy (J) = power (W) × time (s)
Therefore, for a typical LPLD atrial ablation lesion, the energy delivered may be in the order of 900 J (30 W × 30 s), with around 90 J delivered to the myocardium. Energy applied in a high-power, short-duration lesion may actually be significantly less, in the order of 450 J (90 W × 5s), with around 45 J delivered to the myocardium.
Consider next how this energy is converted into heat. Temperatures above 50°C result in irreversible cell death, while those in the range of 45–50°C have been shown to create lesions with reversible injury and therefore the ability to recover excitability.15,18,19 The specific heat capacity of a substance is the amount of energy that must be added to 1 g of that substance to increase its temperature by 1°C, and is a physical constant that varies depending on the physical properties of the substance, in this case atrial myocardium. Temperature change is therefore related to applied energy, mass and specific heat by the following equation:
ΔT = Q / MC
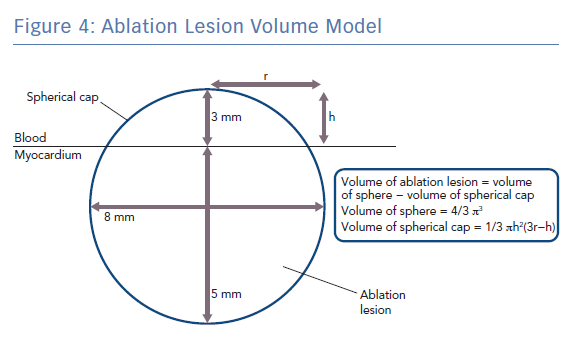
Q is the energy added (measured in joules), m is the mass of the substance being heated (measured in grams) and c is the specific heat capacity of the substance (measured in Jg−1K−1). Specific heat capacity for myocardium is temperature dependent but values around 3.111 Jg−1K−1 have previously been used in simulation studies of cardiac radiofrequency ablation.20,21 Taking the density of myocardium as 1.053 g/cm3, and modelling a typical lesion as a truncated sphere with a depth of 5 mm and a width of 8 mm (volume 0.18 cm3 ; Figure 4), it can be shown that around only ~14 J would be required to raise the temperature of myocardium within the ablation lesion from 37°C to 60°C, which is well within the energy delivered by both typical LPLD and new high-power, high-duration settings.22 Put another way, at generator power outputs of 60 W, where around 6 W is delivered to the tissue, an effective 5 × 8 mm lesion could be formed in around only 2–3 seconds, which is consistent for example with the time taken to eliminate conduction in an accessory pathway that has been accurately located.
Therefore, it is the total quantity of energy delivered to the tissue, not the duration of energy application per se, that is important in achieving tissue heating and ablation lesion formation. Nevertheless, simulation studies show that multiple other factors including electric conductivity, thermal conductivity, current density, electric field intensity and heat loss due to blood perfusion in the myocardial wall all influence ablation lesion formation.21 Although the effects of perfusion in the myocardial wall are minimal, the effects of cooling from myocardial blood flow and catheter irrigation are not negligible, with the result of cooling the tissue surface within the immediate vicinity of the electrode and reducing the lesion size at the tissue surface.23–25 In HPSD ablation, the majority of tissue death occurs via resistive heating and, as a result, theoretical advantages have been proposed, including optimised lesion geometry, reduced collateral tissue damage and increased durability of electrical isolation, in addition to obvious benefits in procedural duration.8,14,17,26 These characteristics are explored in more detail below. However, to understand the optimal duration of high-power energy delivery, it is also necessary to predict the degree of conductive heating that might occur when the duration of energy application is altered. Although there are numerous reports describing the advantages of HPSD ablation, no currently available technology can provide adequate real-time assessment of lesion formation and recommendations for HPSD ablation are based on trial and error experience from highly expert centres.8,14,17,27
Characteristics of High-power, Short-duration Ablation
Lesion Geometry
Studies of lesion geometry at different power and duration settings demonstrate a clear correlation between total energy delivery and lesion depth, and suggest that the total energy delivered is the key variable rather than the rate at which it is delivered.14,17,26,28 There is also a trend towards wider lesions with increasing power. In most of these studies, lesion diameters were generally measured histologically under direct visualisation. Only one study measured the distance from the endocardial surface to the 53°C isotherm, previously shown to indicate irreversible tissue injury, and could therefore include a comment on the contributions of conductive and resistive heating with HPSD ablation.14
A further study performed in vitro on porcine left ventricular myocardium compared lesion geometry at irrigation rates of 2 ml/min and 17 ml/min.26 At a lower irrigation flow, application of higher powers led to steam pops. The higher irrigation flow allowed delivery of higher powers of 40 W for up to 30 seconds compared with 10 seconds at a lower irrigation flow. Although the surface width was reduced with higher irrigation rates, there was little difference in lesion depth.
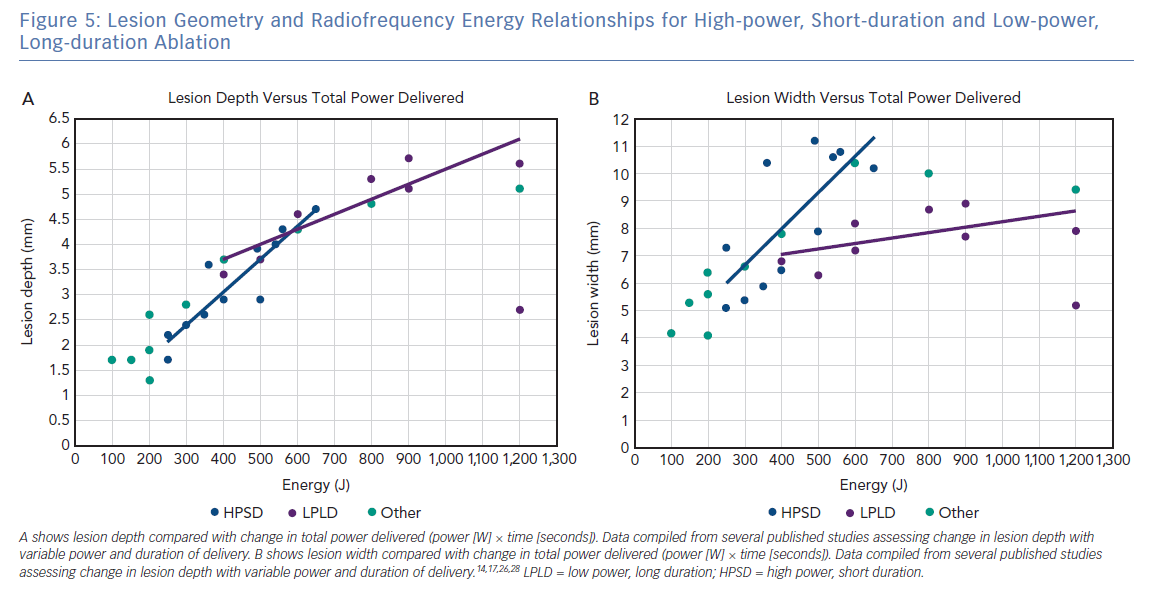
At this time, there is no consensus definition for what constitutes HPSD ablation, making it challenging to describe the clinical settings required to achieve the increased efficacy and improved safety reported. Figure 5 shows the relationship between total energy delivery (J), lesion depth (mm) and lesion width (mm) based on published in silico, ex vivo and in vivo studies. Data points are coloured, based on arbitrary definitions of ablation category as lower power, long duration (mean power 20–39 W, mean duration >20 seconds), HPSD (mean power >50 W, mean duration 0–9 seconds) or other (mean power 40–49 W, mean duration 10–19 seconds). As predicted on the basic principles described above, there is a linear relationship between total energy and lesion depth, regardless of the classification of ablation type. However, the relationship between total energy and lesion width is more complicated, with HPSD ablation resulting in a greater lesion width than lower-power, long-duration ablation for the same energy delivered. This difference likely results from a combination of an increased contribution of conductive heating to lesion formation in lower-power, long-duration ablation combined with increased duration of delivery to permit the cooling effect of endocardial blood flow and/or catheter irrigation to confine lesion width.
Complication Rates and Collateral Damage
Major complications occur in approximately 3.5% of AF ablation procedures because of direct and collateral injury among other factors.29 While there is a vast worldwide experience with conventional ablation settings, the safety profile of HPSD ablation is much less well established. A single retrospective study of almost 14,000 ablations demonstrated low complication rates, but procedure technique varied, with duration of application in a range of 2–15 seconds. There are concerns that high power could increase complications, including steam pops, charring and cardiac perforation, leading to tamponade. Steam pops in particular instigate thrombus formation, which can embolise, causing cerebral ischaemic events. A loose correlation has been illustrated in previous studies between procedure length and risk of developing asymptomatic cerebral ischaemia identifiable on brain magnetic resonance imaging, although there are no current robust data to explore an association between HPSD and the risk of cerebral lesions.30,31
In vitro ablation has previously been performed using a myocardial phantom set-up, comparing conventional settings of 40 W/30 s against several HPSD settings in a range of 40–80 W/5 s. At 70 W/5 s and 80 W/5 s applications, catheter tip temperature sensors measuring above 80°C were shown to have potential for serious complications.32 This was reinforced in vivo by steam pops occurring at both settings. Interestingly, collateral thermal injury to the lungs was observed in conventional settings as well as at 80 W/5 s and the optimal setting found to minimise collateral damage while creating durable lesions was 50–60 W/5 s.14
The relationship between thermal latency and lesion depth using identical radiofrequency energy but over shorter time periods (1 second, 5 seconds, or 30 seconds) has been tested using a computer model. Tissue becomes overheated with temperatures over 100°C at 1 second and 5 second durations and could lead to collateral damage and complications in the clinical setting.33 These findings were correlated in vivo whereby lesions created without temperature limitation resulted in a 1.7% incidence of steam pops, all of which occurred at temperatures over 85°C.17 For this reason, all studies advocating HPSD ablation strongly recommend the use of an automatic temperature cut-off. The exact cut-off varies between studies between 50°C and 80°C.8,14,34–36 Small animal studies have shown safety and efficacy in using high powers between 50 W and 90 W with contact forces of 10–20 g and using irrigated catheters to maintain a catheter tip-tissue temperature of less than 55–65°C during lesion formation.8,14,17,37
Atrio-oesophageal fistula as a consequence of left atrial ablation is a rare but life-threatening complication with a mortality rate of at least 50%. The oesophagus can lie within 2 mm of the posterior left atrial wall, placing it in danger of injury with changes in lesion depth.38 The combined experience to date is too limited to define the risk of oesophageal injury with HPSD ablation. However, late gadolinium enhancement MRI of the oesophagus in 574 patients following AF ablation using user-defined HPSD settings of 50 W for 5 seconds reported a 14.3% incidence of moderate to severe thermal oesophageal late gadolinium enhancement, although no fistulas were reported.35 The patterns of severity were similar between HPSD and LPLD groups despite a marginal increase in comorbidities in the study group, a larger left atrial volume based on volume index and more central positioning of the oesophagus in relation to the posterior left atrial wall. These findings are in keeping with a previous retrospective study of more than 10,000 patients where atrio-oesophageal fistulas were recorded in four patients who underwent HPSD ablation. In three of these patients, power was reduced from 45–50 W to 35 W on the posterior wall and ablation performed for a longer duration.9
From a biophysical perspective, it is conceivable that HPSD ablation may be safer for patients because it causes less collateral damage. Initial computer simulation studies generated concern that delivering a similar amount of energy over a shorter duration would result in lesions of greater depth due to thermal latency after the ablation had been halted.33 This strategy led to significant elevation in catheter tip temperature of up to 100°C when total energy was delivered over 1 second and 5 seconds and remained above 80°C for up to 7–10 seconds after termination of delivery, well within the tissue overheating range.
Further simulations have demonstrated that HPSD lesions require less total energy delivery to achieve wider but shallower lesions which may somewhat mitigate this risk.28 In line with the theoretical predictions described above, experimental data confirms that, with HPSD ablation, the ratio of endocardial heating to irrigation-mediated cooling is higher, reducing convective cooling, mitigating subendocardial sparing and including the endocardium within the maximum zone of heating.17 Furthermore, the shorter duration restricts conductive heating and therefore thermal latency, creating a shallower lesion.28 In fact, earlier studies show a discrepancy in the rates of complications, such as steam pops and charring, with conventional generator settings between in vitro and in vivo experiments, with higher complication rates in vivo. This was thought to be a consequence of poor heat dispersion, which is accentuated by a longer duration of ablation in vivo.14 The suggestion of reduced collateral damage was further demonstrated during an in vivo study where conventional settings showed lung injury and temporary phrenic nerve palsy that was absent in HPSD ablation. However, given the infrequency of such complications, these results must be interpreted with caution until larger, multicentre trials can corroborate or refute these findings.17
Clinical Outcomes and Lesion Characteristics
Several trials reaffirm that the clinical outcomes of AF ablation remain modest.5,39–41 The Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial (CABANA) demonstrated that only 50% of all patients undergoing AF ablation remain free of AF at 3 years after the procedure, with up to 17% requiring further ablation procedures.42 One mechanism for the recurrence of AF following pulmonary vein isolation is the reconnection of the pulmonary veins to the left atrium, which may be because of gaps in the pulmonary vein encirclement or non-transmural lesions within the encirclement.43–45 Incomplete lesion formation can result in reversible conduction block due to transient oedema creating temporary electrical isolation at the time of the procedure.46 Recurrence of arrhythmia may accompany recovery of left atrium to pulmonary vein conduction in the weeks following ablation once inflammation has resolved.47 Anatomical studies have measured the maximum wall thickness of the sleeves surrounding the pulmonary veins at less than 4 mm, with an average thickness of 2 mm.48,49 Durable lines therefore require contiguous thermal injury to this depth to create permanent pulmonary vein isolation.
As described earlier, conventional ablation at LPLD settings relies on a combination of resistive and conductive heating to produce transmural lesions. Mathematical modelling shows resistive heating to achieve a lesion depth of only 1–1.5 mm during LPLD ablation, requiring conductive heating to create deeper transmural lesions.16,18 In contrast, HPSD ablation studies using computer modelling, static tissue and in vivo have demonstrated lesions of greater depth being generated due to resistive heating with minimal conductive heating, a biophysical lesion profile that may result in more predictable lesion formation in the thin-walled atrium (Table 1).14,17,26,28,33,34 When compared with conventional ablation settings, HPSD (90 W/4 s) linear ablation and pulmonary vein isolation therefore resulted in more predictable lesion formation, contiguous lines and transmural lesions in beating pig hearts.17 In comparison, conventional ablation settings resulted in gaps visible to the naked eye, variable lesion sizes and non-transmural lesions on histology.17
Catheter stability is also an important determinant of lesion formation.10 Longer-duration energy applications may be associated with compromised stability of catheter contact. Lesion characteristics determined in ex vivo stationary tissue preparations do not accurately capture the range of movement seen in the beating heart, where increased variability between lesions and an overall smaller lesion size are seen compared to ablation in a stationary muscle tissue preparation.17 Longer application times as a result of reduced catheter contact and stability could conceivably lead to increased local tissue oedema, reversible atrial injury and therefore only temporary pulmonary vein isolation.8 For these reasons, HPSD ablation may result in improved permanent pulmonary vein isolation although this requires investigation in further clinical studies.
Procedural Duration
AF catheter ablation procedure durations vary between operators and centres but typically last between 90 and 180 minutes with point-by-point radiofrequency energy application techniques.9,14 In an era of increasing disease burden, the demand for AF ablations continues to rise with greater numbers of patients of increasing complexity being referred for intervention.1,2,47 With the costs of AF care estimated to amount already to 1% of the UK’s NHS budget, methods of reducing costs and improving resource allocation must be considered.2,3
Against this background, one potential benefit of HPSD ablation might be a reduction in procedure time. In a porcine study, radiofrequency delivery for 5 seconds at 50 W achieved a mean lesion depth of 3 mm, while at 30 W a duration of 2–4 times longer (10–20 seconds) was required to achieve lesions of a similar depth.26 In early clinical studies dating back to 2006, cautiously attempted HPSD ablation with settings of 45 W/20 s was compared with a control group of 30 W/120 s, and published results indicated ablation time was reduced by as much as 80%. This was accompanied by a more modest 26% reduction in overall procedure time.8,37
More recently, the Clinical Study for Safety and Acute Performance Evaluation of the THERMOCOOL SMARTTOUCH SF-5D System Used With Fast Ablation Mode in Treatment of Patients With Paroxysmal Atrial Fibrillation (QDOT FAST) trial was the first prospective clinical multicentre trial of HPSD ablation, performed as a feasibility study. The total procedure time was 105 ± 25 minutes with an average fluoroscopy time of 6.6 ± 8.2 minutes and included a 20-minute waiting period after pulmonary vein isolation and an adenosine/isoproterenol challenge.36 Although there was no comparative control group within the study, this does compare favourably with previous reports on procedure duration using radiofrequency technology.
Particular patient groups such as those with heart failure may benefit from shorter procedural and ablation times. Since both LPLD and HPSD ablation lesions are delivered with irrigation, the duration of radiofrequency energy application is directly linked to the volume of intravenous fluid delivered intraprocedurally. In the QDOT FAST trial, average periprocedure fluid volume delivery was 382 ± 299 ml compared with 898–1,880 ml previously demonstrated in the NAVISTAR THERMOCOOL Catheter for the Radiofrequency Ablation of Symptomatic Paroxysmal Atrial Fibrillation (THERMOCOOL AF), SMART-AF and THERMOCOOL SMARTTOUCH Catheter for the Treatment of Symptomatic Paroxysmal Atrial Fibrillation (SMART-SF) Radiofrequency Ablation Safety Study trials.50–52 Despite this theoretical advantage, however, a clinical benefit of reduced irrigation fluid delivery has not yet been demonstrated. Furthermore, the study size of QDOT FAST Trial (n=52) lacked power to provide conclusive results other than to demonstrate feasibility and the need for further studies. A larger, on-going clinical trial of 185 patients is in progress, the Evaluation of QDOT MICRO™ Catheter for Pulmonary Vein Isolation (PVI) in Subjects With PAF study (Q-FFECIENCY; NCT03775512).
Further Trials
One of the main challenges in radiofrequency ablation is the inability to reliably assess tissue temperature in real time as a marker of lesion formation. The vast majority of published studies describing HPSD ablation involve catheters containing a single thermocouple; however, the catheter irrigation system negates the utility of this thermocouple for the estimation of tissue temperature. Catheter stability and orientation can further confound temperature readings, which are made from a single point of contact.
A novel irrigated tip catheter, the QDOT Micro Catheter (Biosense Webster), has been designed. It contains six thermocouples, three of which are positioned distally and embedded more superficially than previously at 75 μm below a metallic tip that acts as a high-quality conductor between the tissue-catheter interface and the thermocouples. The remaining three thermocouples are 3 mm more proximal enabling accurate temperature recording in real time that is independent of catheter orientation.8,17,27,34 In addition, the catheter retains the SmartTouch (Biosense Webster) technology for contact force sensing and has an improved cooling irrigation system with backward flow towards the most proximal thermocouple. This is designed to reduce temperature-sensing inaccuracies and to increase temperature sensitivity in the parallel catheter orientation, which has previously been challenging.17 The catheter can be used alongside a proprietary radiofrequency generator that has a short ramp-up time of <0.5 seconds, which, when combined with temperature feedback every 33 milliseconds, allows finer control of delivery during applications.53 Further work is needed to determine optimal ablation settings for HPSD ablation.
A mathematical model has been proposed incorporating temperature measurements, contact force sensing, impedance and catheter orientation, which aims to enable accurate lesion size prediction with the use of the QDOT Micro Catheter. In a swine study, this model demonstrated a strong correlation with lesion depth with a prediction error of 1.5 mm between lesion depth estimates and those measured with histology.27 Considering the theoretical predictions discussed above, it is unsurprising that lesion depth is predictable given the direct correlation between energy delivery and lesion depth. However, it is expected that there could be a different relationship with lesion width in view of other variables that affect ablation lesion formation, including blood flow and tissue surface cooling. Such phenomena may be especially important to lesion contiguity during HPSD ablation and require further study, as has been done for conventional ablation.54
The QDOT Micro Catheter was subsequently trialled using a thigh muscle tissue preparation to simulate cardiac ablation. A generator output of 90 W delivered for 4 seconds was identified as the optimum setting that would provide high power at short duration and with low risks of steam pop or thrombus formation. These settings were subsequently used to perform cardiac ablation in 15 swine to assess safety and lesion durability, which were respectively found to be superior and equal to conventional settings.17 A further small study by the same group found that all posterior lines created in the right atrium with HPSD ablation were wider and remained intact after 30 days while none of the lines performed at conventional settings remained intact.8 Despite seemingly encouraging results, it must be acknowledged that these studies may not be directly transferable to clinical use, particularly given the narrow window between therapy and safety at high power. The QDOT FAST trial is the only trial to have attempted very high power in clinical use and, despite a small cohort, a significant adverse event did occur, with one patient diagnosed with a haemorrhage from an oesophageal ulcer that occurred one day after the procedure which was managed medically.
Gaps in Knowledge
Much of the literature to date shows promise for the use of HPSD ablation but there is no consensus on the precise power and duration that conveys the maximum potential clinical benefit with the least possible risk. Current definitions for HPSD ablation vary from 50 W to 90 W for durations of 2–20 seconds. The majority of human trials to date have used a maximum power of 50 W for perceived patient safety reasons, but recent findings suggest that it might be possible to use higher generator power outputs in patients. Further research is therefore required to ascertain the optimal generator settings for human AF.
A clinical trial of HPSD ablation using the QDOT Micro catheter is on-going in a cohort of 185 patients. Outcome measures include procedure efficacy measured at the time of the procedure, within 7 days and up to 12 months after ablation and early adverse events within 7 days of the procedure or significant adverse events within 30 days. The results of this trial will be informative in clarifying the safety profile of HPSD ablation in patients.
A better understanding of lesion geometry created using HPSD ablation is also required to understand the balance between resistive heating and conductive heating as power applied is increased. This will aid in predicting changes in lesion formation with variable power delivery and duration of individual lesions. At a time when there is an increasing shift towards individualised patient therapies, more in-depth knowledge of lesion geometry may allow titration of ablation settings to the target, for example based on atrial wall thickness or areas of low voltage.55
Of note, sample numbers from trial data so far are small. There have been few prospective or multicentre trials, and no randomised trials at this time. Larger, randomised controlled trials assessing high and very high power delivery will be essential to determine the efficacy and safety of these settings. Given the infrequency of major complications such as stroke, pulmonary vein stenosis and atrio-oesophageal fistula formation, it is likely that a true understanding of patient safety will be difficult to fully delineate from small randomised trials. A clear advantage in safety, efficacy and cost must be indisputable to advocate a change to HPSD ablation given the vast quantity of data collected from hundreds of thousands of patients worldwide that has formed current ablation techniques.
Clinical Perspective
- Successful AF ablation therapy is contingent on the creation of durable, contiguous transmural lesions that result in permanent pulmonary vein isolation.
- High-power, short-duration (HPSD) ablation demonstrates promise as an alternative approach to radiofrequency energy delivery, potentially contributing to superior lesion formation, non-inferior complication rates and shorter procedural times.
- On-going clinical trials using the novel QDOT Micro Catheter will gather data to assess the safety and efficacy of HPSD ablation. At present, QDOT-FAST is the only prospective, multicentre trial assessing HPSD ablation.
- Much more extensive validation of the use of HPSD ablation will be required before widespread uptake can be recommended.