Cardiac implantable electronic device (CIED) implantation is increasing worldwide. In 2013, in the EU, the implantation rate for cardiac pacemakers was 532/million population and for ICD was 100/million population; furthermore, more than 51,000 CRT devices were implanted.1 In the US, pacemaker implantation rates increased by more than 50% between 1993 and 2009.2
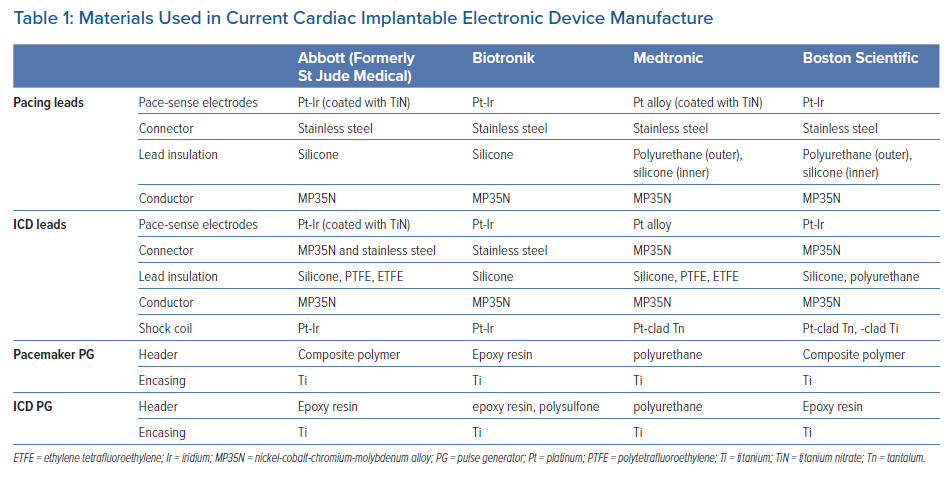
The main components of a CIED in contact with the patients tissues are the pulse generator encasing (made from titanium), the connector head (made from a polymer) and the leads.3 The materials used to make each of these components varies slightly depending on the manufacturer and type of device.
Hypersensitivity reactions (HSR) to metals and epoxies have been reported in orthopaedic, vascular neurosurgical and interventional cardiology in the form of coronary and vascular stents and endovascular occluder devices.4–7 HSRs to components of CIEDs are thought to be a rare complication. However, while the true incidence is not known, it may be as high as 1/500 cases.8 The pathophysiology of HSRs to components of CIEDs involves delayed hypersensitivity (type IV), as suggested by granulomatous dermatitis recovered from biopsy specimens and may develop without any prior allergen exposure.9 HSRs can occur in various degrees of severity, from localised skin erythema and urticaria to generalised systemic symptoms and – in extreme cases – even anaphylactoid reactions. Differentiating HSRs from device infection (a much more common complication of CIED implant) is challenging and can lead to delayed diagnosis, unnecessary antibiotic treatment and complex device extraction procedures, potentially resulting in increased morbidity and mortality.10
The aims of this systematic review are to summarise the available literature on the aetiology, diagnosis and management of HSR in CIED patients and to provide guidance on the best management strategies in these patients.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol for systematic review guidance was followed.11 We systematically searched for publications written in the English language on HSR to CIED in PubMed from January 1970 to November 2022. The words “pacemaker,” “cardiac implantable electronic device,” “cardiac rhythm device,” “implantable cardiac defibrillator,” “cardiac resynchronisation therapy” or “cardiac device” in combination with “allergy,” “dermatitis,” “contact sensitivity,” “contact dermatitis,” or “hypersensitivity reaction” were used as search criteria. Further case reports were found from the references of the articles found from the search criteria above. Case reports, case series, reviews and editorials pertaining to allergic reaction to a CIED were screened. We collected data on basic demographic characteristics (age, sex, prior history of HSR), CIED type and manufacturer, symptoms (type: local or systemic, and clinical description), diagnostic tests (patch testing and their results, blood tests, blood cultures, histological examination of tissue samples), time from implantation to symptoms and diagnosis, number of interventions required to reach diagnosis, treatment used and its efficacy and duration of follow up. Data collection was carried out independently by two reviewers, and any discrepancies were discussed and consensus reached. The robvis tool was used to assess the risk of bias.12
To identify the materials used in the manufacturing of current CIED, we checked the product manuals from the most commonly implanted CIED manufacturers (Abbott, Medtronic, Biotronik and Boston Scientific). All companies are currently using titanium for encasing the pulse generator of their CIEDs. Lead conductors are made of nickel-cobalt-chromium-molybdenum (MP35N) alloy, which is covered with a biologically inert material (silicone or polyurethane). The lead pace/sense electrodes are made of platinum alloys, while the ICD coils are made of platinum alloy or tantalum.13–17 Table 1 provides a summary of materials used in current CIEDs.
The systematic review was registered with PROSPERO (international prospective register of systematic reviews), registration number CRD42021227653.
Categorical data are expressed with counts and percentages (%), and continuous variables as average ± SD. Where appropriate, comparison between groups was performed using Mann-Whitney test for continuous variables, and Fisher’s exact test for categorical variables. A p-value of ≤0.05 was considered significant.
Results
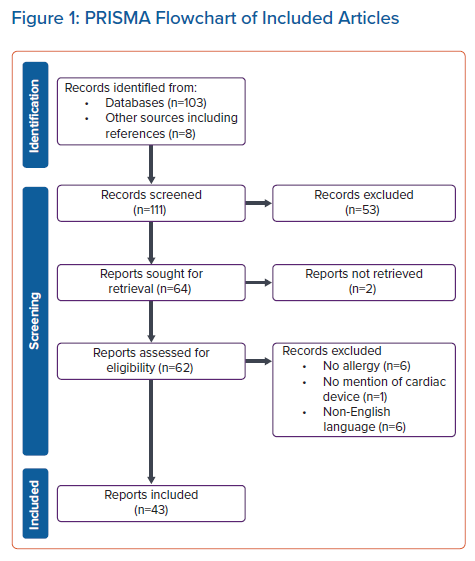
Figure 1 shows the PRISMA flow-chart of included studies. We screened 111 publications (103 primary publications and eight additional publications identified from the primary publication reference lists). From these, we excluded 68 publications for the following reasons: not meeting the inclusion criteria (n=53), inability to access (n=2), no allergic reaction reported (n=6), no mention of CIED (n=1) and non-English language (n=6). Following these exclusions, 43 publications were included in the systematic review, reporting on 57 individual cases.
Quality of Evidence and Risk of Bias
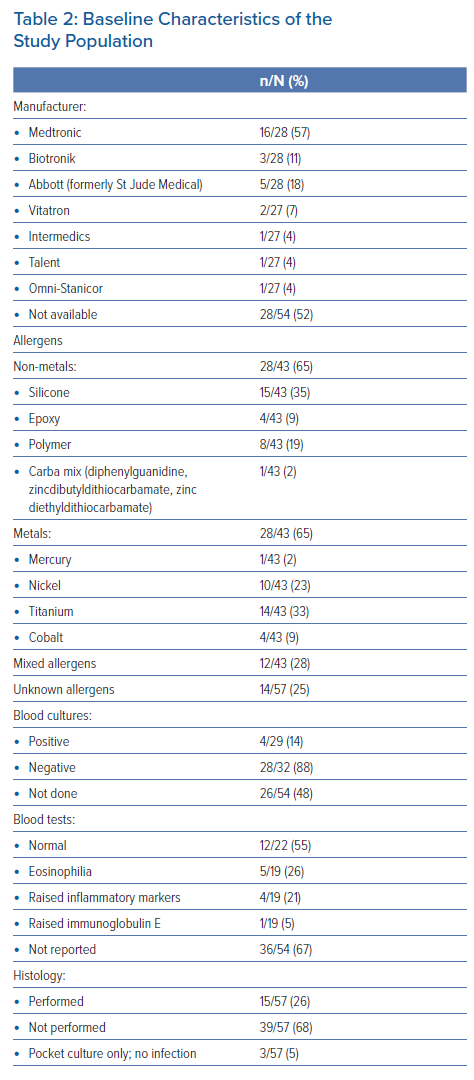
The quality of evidence was assessed as low, due to lack of consistency in diagnostic criteria, assessments and treatments applied, imprecision of assessments and publication bias. Data on age, type of device, allergens, blood tests, cultures and histology were frequently not reported (Table 2).
Baseline Characteristics
Of the cases identified, the majority were permanent pacemakers. However, there were reports of HSR to ICDs and CRTs. Two patients had HSR to epicardial systems with abdominal generators, and one was an externalised temporary system. There were 11 cases from Gold et al. where the type of device from each individual case was not specified.18
The mean age was 57 ± 21 years (range 9–87 years), and 48% of patients were women. Age and sex were not reported in 13 patients (23%). Previous allergy status was documented in only 14 patients (25%) with 10 having a history of allergic reactions to various substances (Supplementary Material Table 1).
Symptoms
The time taken to develop symptoms from implantation of CIED was 29 ± 59 months (range 1 day to 10.5 years). Symptoms included local (44/57 patients, 77%) and systemic reactions (12/57 patients, 21%); four patients (7%) had both. Localised reactions included contact dermatitis, localised erythema, pruritus, vesicles or swelling. Systemic reactions included a generalised dermatitis in 5 patients, chest pain and shortness of breath, a pompholyx reaction on the hands, eosinophilic myocarditis, widespread nummular eczema, anaphylactoid reaction, widespread erythroderma, fever, and deterioration of the patient’s asthma.18–27 Interestingly, one case reported pacemaker malfunction, resulting from the development of a serous fluid pocket that seeped into the connector site causing excessive current drainage and early battery depletion; the patient presented with recurrent syncope because of no output.28 In addition, one patient was known to have a titanium allergy and was implanted with a gold coated pacemaker at the index procedure.29 Full descriptions of presenting reactions are detailed in Supplementary Material Table 1.9,18–59
Diagnosis
The time of initial symptoms to diagnosis was 33 ± 51 months (range 2 days to 9 years). Diagnosis was delayed in 45 patients (79%). Patients frequently underwent multiple CIED system extractions before a diagnosis was made (average 1.7 ± 1.3 procedures/patient; range 0–6).
Of the 57 cases, 53 (93%) had patch tests to identify the allergen. The identified types of allergens are summarised in Table 1. Multiple allergens were identified in 11 patients (19%). In 14 cases (25%) no allergen was identified, either because the patch test was negative or the patient was unable to undergo patch testing.
Only 31 patients (54%) had blood cultures performed; in three patients (11%) they were positive.18,33,39 In two of those patients, the isolation of an organism was interpreted by the authors as being ‘a red herring’ because the patients underwent multiple extractions and the symptoms only settled after the allergen was identified and either removed or covered during implant. This is because the patients underwent multiple extractions and the symptoms only settled after the allergen was identified and either removed or covered during implant. In one report, the authors suggested that the device infection was secondary to wound dehiscence due to an allergic reaction. Results of blood tests were reported in 22 patients and were normal in 12 (55%). Eosinophilia was seen in five patients (23%), raised inflammatory markers in four patients (18%) and raised immunoglobulin E in one patient (5%). Histology results from tissue collected from the CIED pocket were reported in 15 patients and are detailed in Supplementary Material Table 1. The histology results described foreign body granulomas, spongiosis and lymphocytic infiltration. In one patient with eosinophilic myocarditis a myocardial biopsy was performed. In six patients (including three who also had histology samples), cultures from the pocket were taken and the results were negative.
Treatment
Treatments applied to both local and systemic reactions included steroids, explantation of device with or without reimplantation of devices coated in a non-allergenic material or explantation and reimplantation of similar devices in conjunction with oral steroids. Treatment success varied. For local reactions, topical steroid treatment without device removal had limited efficiency (4/7 cases, 57%). Explantation and reimplantation of a device coated with a non-allergenic material was usually successful (gold in 6/6 cases, polytetrafluoroethylene [PTFE] in 8/8 cases and titanium in 1/1 case), but the numbers of reported cases is small and the reported follow-up duration was below 2 years in a majority of cases, except one report with a 9 year follow-up.26 In patients with silicone allergy, implantation of a silicone-free device had limited efficiency (3/5 cases, 60%). In one case, anti-histamine drugs were used to treat local reactions, but the efficiency was not clearly reported.
For systemic reactions, steroids without device removal had very low efficiency (1/3 cases, 33%). The preferred treatment method was explantation with device reimplantation. However, details on the type of device and coats used for reimplantation and their success rate was not consistently reported (Supplementary Material Table 1).
Discussion
This systematic review summarises the available English-language literature regarding HSRs to CIED. The findings can be summarised as follows: first, even if HSR is assumed to be a rare occurrence, the true incidence is unknown, and likely under-reported; second, diagnosis is frequently delayed as HSR can masquerade as device infection; third, virtually any component involved in the manufacture of CIEDs that is in contact with tissue has been reported to potentially cause HSR; fourth, topical or systemic treatment with steroids has limited efficiency in treating HSR to CIED components, and there are not enough data to support the effectiveness of this treatment; and fifth, the preferred treatment is full device removal, followed by reassessment of indication for CIED and – if indicated – reimplantation of devices coated in non-allergenic materials. When a HSR to silicone has been reported, reimplantation of silicone-free devices seem to have low efficiency. However, these conclusions have to be interpreted with caution due to low quality of the data.
The mechanism of a HSR to components of implantable devices is a delayed hypersensitivity reaction (type IV), mediated by T-cell activation.7,9 The most common sign of HSR is a diffuse pruritic eczematous rash around the site of device implantation. However, HSR can present with a wide array of symptoms. In cases of HSR to intravascular and coronary stents, they may present as instant stenosis. In cases of cardiac occluders, a systemic reaction described as ‘Kounis syndrome’ or ‘device syndrome’ has been reported.60 In neuroendovascular intervention or intracardiac devices, allergic reaction may cause embolic events as it forms a nidus for thrombus.61 In patients with a CIED, the reported symptoms of HSRs are varied and non-specific, and they are difficult to differentiate from CIED infection. The latter is a frequent and severe complication of CIED implantation with a reported average incidence of 1–2%, depending of CIED type.62 CIED infections universally require full CIED extraction, as conservative treatment has unacceptable failure rates.63 For all practical purposes, given the poor quality of the data on HSR to CIED available in the literature, any local or systemic reaction in patients with CIED should be considered as indicating infection and not HSR until proven otherwise, and relevant guidelines followed; HSR should not be the first diagnosis. This prudent approach is based on the following points:
- In a period of 17 years with 4,497 implants, Yashiro et al. reported an incidence of 2.4 per 1,000 procedures.8 Other groups have identified only three HSR cases out of more than 5,500 implanted devices (incidence <0.5/100 procedures).26 Thus, even if the true incidence of HSR is unknown and the cases are likely under-reported, it is reasonable to conclude that CIED infections have an approximate 5- to 10-fold increased incidence compared with CIED HSR.8 Even though the estimated of risk of HSR to CIED is low, it is advisable to inform the patient regarding this potential complication during the informed consent process, because of the significant associated morbidity. It is also advisable to document any allergies, which would give the patient and clinician a better understanding of the potential risk of HSR and may potentially mitigate this risk upfront.
- Only half of the patients reported in the literature having HSR to CIED had blood cultures taken, and in four of these the cultures were positive. In addition, tissue samples were only analysed in less than a quarter of cases. As such, even in some cases included in this systematic review, CIED infection cannot be definitively ruled out. On the other hand, patch testing was also not routinely performed, so doubts regarding the correct diagnosis of HSR can be raised in many cases. The picture is further complicated by the fact that patch testing has relatively low sensitivity in diagnosing CIED HSR.
- The treatment of choice for both CIED infection and HSR is device removal. Conservative approaches have very high failure rate and should only be considered in very frail patients where the risks of device removal are prohibitive.
Clinical evaluation and patch testing for HSRs to metals before CIED implantation is of limited use and is not recommended. The metal used for the pulse generator box in CIEDs is almost exclusively titanium (>99.9% purity), but diagnosing HSR to titanium is difficult. Yamauchi et al. demonstrated that using intracutaneous and lymphocyte stimulation testing by incubating titanium in the patient’s serum prior to intracutaneous injection can demonstrate titanium sensitivity in a patient whose patch test to titanium was negative.23 Also, a non-systematic review by Fage et al. concluded that currently there is no reliable form of testing for titanium allergy.64 Furthermore, the titanium test is unreliable because this test is performed using titanium tetrachloride, which must be highly diluted with water and quickly hydrolysed to insoluble titanium dioxide.9
Nickel is a far more allergenic metal than titanium, and a positive reaction to nickel can be found in up to 20% of the population.65 National and international standards have been put in place to limit nickel skin exposure at a population level (for example, the European Union Directive on Registration, Evaluation, Authorisation and Restriction of Chemicals). They propose a limit for nickel release of <0.5 μg/cm2/week for items intended to be in direct and prolonged contact with the skin, and <0.2 μg/cm2/week for items pierced into parts of the body (e.g. ear rings).66 We are not aware of legislation limiting nickel exposure in medical devices. In patients with confirmed nickel hypersensitivity, there is a 2.6-fold increase risk of adverse outcomes following insertion of a nickel containing endovascular device, but the presence of skin nickel HSR does not necessarily imply development of HSR to implants containing nickel.6 For cardiology application, nickel HSR seems to be more of a concern for cases of endovascular devices made of nitinol (an alloy made of 55% nickel and 45% titanium) such as stents or occluders.6 The clinical picture has been described as Kounis or device syndrome, although controversies still exist regarding its incidence, pathogenesis and even existence.60,7 For CIEDs, the real impact of nickel HSR is even less clear. This is because in CIEDs, nickel is found only in the manufacturing of lead conductors, as part of nickel-cobalt-chromium-molybdenum alloy, which contains 35% nickel. However, the conductors are covered with a bio-inert material, while the pace/sense electrodes and the ICD coils are made of platinum alloys or tantalum and contain no nickel.13–17 Thus, the nickel-containing CIED lead conductors should be isolated from the patient’s immune system. Indeed, even some cases of CIED HSR attributed to nickel may have been, in fact, titanium HSR, as is case number 3 in Robledo-Nolasco et al., where isolating the titanium pacemaker pulse generator in PTFE coating resulted in excellent outcomes at 9-year follow-up.26 Whether exposure of the metallic conductor through lead insulation breaches increases the risk of nickel HSR is not known, but the risk appears very low.
Thus, CIED HSR remains a diagnosis of exclusion of infection.60 Diagnostic criteria for HSR to metals have been published, but their diagnostic accuracy remains to be defined.67 A high index of suspicion is required in patients with systemic allergic reactions, hyper-eosinophilia and recurrent local reactions in spite of previous CIED removal for presumed device infection with negative blood cultures and negative cultures from CIED pocket samples. In these cases, extensive patch testing for various allergens (both metals and non-metals) should be performed, but negative results do not rule out HSR.10 Following device removal, the strength of indication for CIED should be re-evaluated, and when reimplantation of CIED cannot be avoided, it is prudent to choose devices coated in non-allergenic materials. In cases of allergy to metals or when allergens have not been identified, options may include gold coating, paralyne C (a highly inert, biocompatible polymer) or PTFE sheaths.8,26,42 Indeed, in a cohort of nearly 4,500 patients with implants over 17 years, PTFE was used in 19 patients for both primary and secondary prevention of allergy with no reports of reactions to PTFE.8
Limitations
A previous review on HSR associated with endovascular devices included seven other reports not included in our review (due to non-English language and/or inability to access).10 Nonetheless, only very limited data are available regarding diagnosis and management of HSR related to CIED implantation and the quality of data is low. Consequently, there is intense need for further research in this area.
Conclusion
HSR to CIED components is assumed to be a rare complication of CIED implantation, but the true incidence is unknown and likely under-reported. The diagnosis is frequently delayed as HSR can masquerade as CIED infection. From the limited data available, we can reasonably conclude that the treatment of choice is full CIED removal, reassessment of indication for CIED and reimplantation of devices coated in non-allergenic materials where HSR is suspected. Topical or systemic treatment with steroids have limited efficiency and should not be used. There is an urgent need for further research in this field and an international registry for reporting HSR to CIED would be a welcome and worthwhile initiative. This would improve the diagnosis, investigation and management of HSR to CIEDs.
Clinical Perspective
- Hypersensitivity reactions (HSRs) to components of cardiac implantable electronic devices (CIEDs) is assumed to be a rare condition; however, the true incidence is unknown, and likely under-reported. The diagnosis of HSRs to CIEDs is frequently delayed as HSRs can masquerade as device infection.
- Virtually any component involved in the manufacture of CIEDs that is in contact with tissue has been reported to potentially cause HSRs.
- Diagnosis and treatment of HSRs to CIEDs can be challenging. Topical or systemic treatment with steroids has limited efficiency, and there are not enough data to support the effectiveness of this treatment. The preferred treatment is full device removal, followed by reassessment of indication for CIED and – if indicated – reimplantation of devices coated in non-allergenic materials. However, when HSR to silicone has been reported, reimplantation of silicone-free devices seems to have low effectiveness.