Leadless devices are the next frontier in the field of cardiac pacing. While conventional pacing systems have a long history of widespread use and a robust evidence base, leadless technology is attractive in its potential to subvert the long-term issues inherent to transvenous leads, such as fracture (1–4%), tricuspid regurgitation (5%) and infection (1–2%).1 The first leadless right ventricular (RV) pacing device, the Nanostim (St Jude Medical, now Abbott) was implanted in 2012.2 Roughly 1,400 devices were implanted worldwide; however, it was discontinued due to issues with premature battery depletion.3 The vast majority of knowledge and experience in leadless pacing is through widespread use of Micra (Medtronic), which was first implanted in 2013 (Figure 1).4 There have been more than 150,000 Micra implants worldwide since then, primarily driven by high penetrance in the US, which accounts for over 90,000 implants.5 The EMPOWER device (Boston Scientific) and the Aveir device (Abbott) have entered investigational trials, with the potential to greatly expand the indications for leadless technology.6,7 In addition, the Wireless Stimulation Endocardially (WiSE) for cardiac resynchronisation therapy (CRT) system (EBR Systems) has emerged as a system to effectively provide left ventricular (LV) endocardial pacing in patients with an indication for CRT.8
In this article, we will first review the data for the safety and performance of leadless RV pacing devices, including addressing the topic of atrioventricular synchrony. We will then examine the potential uses of leadless technology in special populations: patients with high infection risk, patients on haemodialysis and those requiring CRT. We will review the challenges associated with leadless pacing, particularly how to manage devices at the end of battery life. Finally, we will provide a perspective on future directions in the field, including the potential for leadless conduction system pacing (CSP) and completely leadless therapy (CRT-D).
Safety
Real-world safety data in leadless pacing is primarily from the Micra-IDE study and the Micra Post-Approval Registry.9,10 The Micra-IDE study was an international multicentre trial of 725 patients who underwent an attempted Micra implantation, with the primary endpoint being freedom from procedure- or system-related complications.9 There were a total of 28 major safety events in 25 patients, a complication rate of 4%. There were 11 cases of traumatic cardiac injury, that is, perforation or effusion (1.6%) and five cases of vascular complications at the groin puncture site (0.7%). This study also evaluated Micra safety against a historical control cohort of 2,667 patients who had received transvenous pacemakers and found that the study patients had fewer major complications (4% versus 7.4%, HR 0.49, 95% CI [0.33–0.75]; p=0.001), fewer hospitalisations and fewer system revisions, with no difference in tamponade rate between cohorts. The Micra Post-Approval Registry of 795 patients reported a major complication rate of 1.5%, including one traumatic cardiac tamponade (0.13%) and six vascular site complications (0.75%), thus demonstrating an improved safety profile for the implant procedure following the initial learning curve.10 These studies support the safety data from the LEADLESS II IDE study.11 This reported a short-term complication rate of 5.8% among 718 patients following Nanostim implantation, superior to a matched cohort who had received transvenous pacing (5.8% versus 9.4%, p=0.01), largely due to the elimination of pocket- and lead-related complications in the leadless cohort. However, in this study there was a higher rate of pericardial effusions in the leadless group (1.53% versus 0.35%, p=0.005) and, importantly, three out of seven of these patients required surgical intervention, suggesting that the severity of cardiac perforation may be greater with Nanostim than with transvenous leads. A large meta-analysis incorporating both the Micra-IDE and the Micra Post-Approval Registry sought to compare effusion rates with those receiving conventional pacing.12 This analysis reported a similar result to the LEADLESS-II study, with a weighted mean effusion rate of 0.31% in the conventional pacing group versus 1.52% in the leadless group.
A post-hoc analysis of Micra trials and registries by Piccini et al. identified several risk factors for peri-procedural effusions, which were similar to those that increase risk in conventional pacing and include increasing age, BMI <20, being a woman, heart failure, prior MI, chronic obstructive pulmonary disorder, absence of prior cardiothoracic surgery, and dialysis.13 The authors developed a risk score for prediction of effusions after leadless pacing and reported that in low-, medium- and high-risk patients, the effusion rates were 0.4%, 1.5% and 4.8%, respectively. Importantly, for medium- and high-risk patients there was a strong association between number of Micra deployments and observed effusion rate, whereas this was not seen in the low-risk group. They suggest from these data that implanters should exercise significant caution when considering redeployments for issues, such as high thresholds in moderate- or high-risk patients.
Overall, it is likely that the effusion rate for leadless pacemakers will continue to be higher than conventional devices, although recent studies would suggest that this risk is reducing following the global learning curve. In the most extensive cohort study to date involving 7,821 patients, the rate of effusion requiring intervention was 1%.14 It is unclear whether this is likely to decrease further with time. The elimination of pocket- and lead-related problems, however, means that it is likely that leadless pacing will outperform conventional pacing in terms of all-cause complications in the long term.
Performance
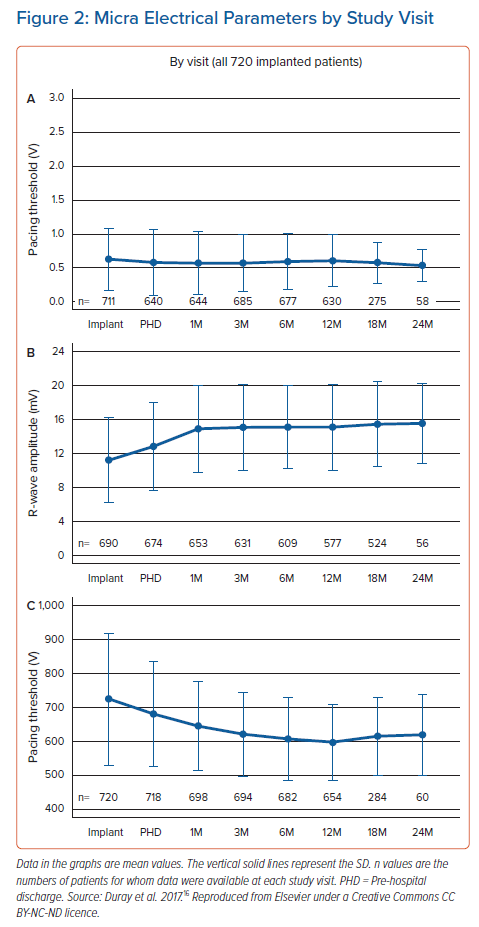
In terms of early electrical performance, in the Micra Post-approval Registry, among 701 patients with available implant data, 87.2% had a pacing capture threshold ≤1.0 V and 97.0% had a pacing capture threshold ≤2.0 V (mean = 0.6 ± 0.5 V at 0.24 ms).9 Results from the Micra Transcatheter Global Clinical Trial suggest that the medium- to long-term device performance was good, with 87% of patients with high implant thresholds (>1 mV) demonstrating an improved threshold at 6 months.15 Duray et al. reported excellent electrical performance of a cohort followed up over 24 months, with reducing capture thresholds, stable R-wave amplitudes and impedance (Figure 2), as well as good battery performance (mean projected battery longevity at 12 months was 12.1 years).16
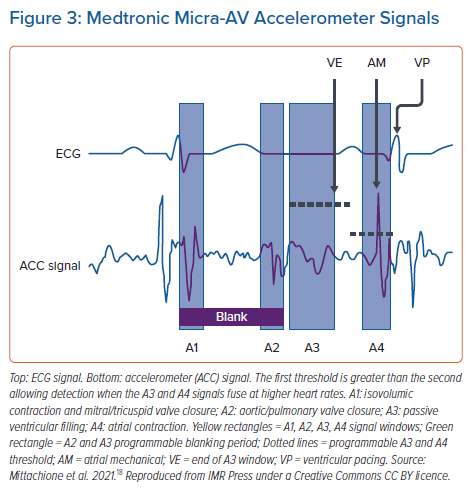
An area of significant interest with regards to electrical performance in leadless pacing is atrioventricular (AV) synchrony and rate responsive pacing. The next generation Micra-AV system aims to provide AV synchrony through the mechanism of accelerometer-based atrial sensing.17 The accelerometer in the Micra device is able to detect various components of the cardiac cycle (Figure 3): isovolumic contraction and mitral/tricuspid valve closure (A1); aortic/pulmonary valve closure (A2); passive ventricular filling (A3); and atrial contraction (A4).18 The MASS and MASS 2 studies tested the feasibility of an algorithm using the detected A4 signal as an atrial marker triggering a programmable AV delay before ventricular pacing.17 These paved the way for the MARVEL study, which tested the feasibility of this single-chamber atrial synchronous ventricular pacing in 64 patients implanted with the Micra device.17 This prospective, non-randomised, multicentre clinical feasibility trial showed an average AV synchrony of 87% (95% CI [81.8–90.9]), with 83% of patients displaying AV synchrony rates over 70%. Importantly, in patients with AV block and those with an intrinsic rhythm, the rate of AV synchrony was lower during fast walking compared to at rest, suggesting that the algorithm underperforms when R-R intervals are short. The MARVEL 2 investigators sought to further delineate the predictors of poor AV synchrony and found in their study of 64 patients that the A4 amplitude was related to the atrial function measured through echocardiography.19 They also report a multivariable analysis showing that high AV synchrony was predicted by an E/A ratio of <0.94 and low sinus rate variability at rest. The authors concluded that these findings may inform future patient selection of VDD leadless pacing systems.
Further work is clearly needed in this area. The MASS and MARVEL studies were conducted with small patient numbers and used the first-generation Micra system with a downloaded AV algorithm, rather that the Micra AV system, for which data is sparse given its recent arrival on the market. Patient selection may ultimately be a key issue here – for example, given reduced AV synchrony at higher heart rates, while Micra may be a suitable option for an ‘average’ more sedentary complete heart block patient, the technology may not yet be at a stage to support the exercise tolerance of more active patients, for example those with congenital complete heart block or sinus node disease.
Preclinical animal studies have demonstrated the feasibility of both a Micra atrial device and the Aveir atrial device, which are still undergoing investigational trials.20,21 Safety profile will need to be closely examined in these devices – the right atrial appendage is a much thinner walled structure than the interventricular septum and RV apex, and as such, the risk of perforation will need to be mitigated. It is the eventual emergence of these dedicated atrial devices which will pave the way for true dual chamber pacing with the potential to match transvenous devices in performance and reliability.
Special Populations
Leadless pacing is becoming a popular choice for patients who require VVI pacing, especially in the US. There are other cohorts of patients for whom leadless pacing may be particularly beneficial, such as those with device infections, haemodialysis patients, and those with vasovagal syncope.
Device infection occurs at an incidence of about 0.5% at primary implantation and 1–7% for secondary procedures, such as generator changes, lead revisions and upgrades.22 Device infection is associated with significant morbidity, with consequences, such as endocarditis, and as such, is a class 1 indication for complete system extraction, which can be a high-risk procedure, especially in the presence of older transvenous leads.23 Leadless pacing is an attractive option in patients with previous device infections, or those who have a higher risk of infection. Autopsy studies have demonstrated complete encapsulation of leadless devices, which theoretically reduces risk of bacterial seeding to the device.24,25 In addition, it has been postulated that both the reduced surface area of leadless pacemakers compared to transvenous leads, and their position in the RV, a high velocity part of the circulation compared to the upper limb venous system, means that device infection is less likely.26 Real-world data in this regard is from analysis of the Micra-IDE study and the Post-approval Registry.26,27 Among 720 patients in the Micra-IDE study, 16 patients had 21 serious infection events during a mean follow-up of 13 months.26 The authors reported that in this small series, no persistent cases of bacteraemia were identified after antibiotic cessation during follow-up, and suggest that systemic infection should be treated differently in the presence of leadless devices compared to transvenous devices, where extraction is strongly recommended in the latter. Sub-analysis of the Post-approval Registry has provided evidence to support the use of Micra following device extraction for infection.27 In this analysis, 105 patients underwent Micra implantation at <30 days following a system explant for infection and 37% of Micra implants in this cohort took place at the same sitting as prior device extraction. The implant success rate was 99%, and during a mean of 8 months follow-up, there were no instances of leadless device explant due to infection. The authors concluded that while larger prospective studies are needed, leadless pacing is a safe alternative for those with device infection who undergo extraction.
Another area where leadless pacing may be the safest – and in some cases, the only available option – is for people who are having haemodialysis. Patients with end stage renal failure requiring haemodialysis represent a high-risk cohort for transvenous pacing systems. Often, indwelling dialysis catheters using the subclavian veins may lead to stenosis or occlusion, thus precluding lead implantation.28 Similarly, pacemaker-induced central venous stenosis can cause multiple issues in patients undergoing haemodialysis, either via reducing central venous options for dialysis catheter placement, or symptomatic stenosis in those with arteriovenous fistula ipsilateral to the pacemaker site.29 The presence of indwelling catheters or fistulas that undergo constant access also increases the risk of seeding to transvenous devices.30 In an analysis of Micra trials, El-Chami et al. examined the outcomes of 201 haemodialysis patients who underwent Micra implantation.31 Of these, 72% had a condition that the physician felt precluded a transvenous device. The implant success rate was 98%, with reasons for procedure failure including inadequate thresholds (1%) and pericardial effusion (1%). There were three procedure-related deaths (two cardiac perforations, one metabolic acidosis following a prolonged procedure where concomitant AV node ablation was undertaken). There were no cases of device-related infection at a mean 6-month follow-up. The conclusions drawn from this study were that the procedure had an acceptable safety profile in this population, albeit in a small study with a short follow-up duration. A re-analysis of the Micra Registry would be useful to validate these findings with a larger patient cohort.
Finally, patients with vasovagal syncope may benefit from leadless pacing for very different reasons. Pacing in vasovagal syncope has an evidence base which consists largely of small, non-blinded studies and have returned mixed results.32 As such, the recommendation for pacing in this indication has been downgraded from 2a to 2b in the most recent guidelines.23 Nevertheless, it is not uncommon in clinical practice to pace those with severe cardioinhibitory vasovagal syncope, especially in the presence of significant pauses. In these patients, who tend to be younger and have a good prognosis regardless of whether treated with pacing or not, leadless pacing offers the ability to provide this therapy without the significant co-morbidity associated with having a transvenous device from a young age. In addition, in view of low pacing burdens, the projected battery life is very long. The evidence in this area thus far is scarce – a retrospective observational study of 32 patients (mean age 37, average of four syncope episodes per year), demonstrated that at mean 404-day follow-up, 87% of patients were free from symptoms.32 These efficacy results are subject to the same caveats of previous trials in this field: lack of blinding, short follow-up and a small number of participants. Given that the primary benefit in leadless pacing here is improvement in lifetime risk profile, observational studies with longer-term follow up, in the region of 5–10 years, will be useful in guiding whether devices, such as Micra, should become a standard of care in selected patients in this cohort where the decision to implant a pacing device has been made.
Leadless Cardiac Resynchronisation Therapy
Not only has CRT become a widespread treatment for dyssynchronous heart failure in the past two decades following seminal trials demonstrating improved mortality in these patients, but the indications are ever expanding, with CRT having now shown benefit in patients with moderate LV dysfunction and a high RV pacing burden and in those who have had AV node ablation.23,33–35 With increasing indications, there has also been an expansion of the cohort that may require leadless CRT. Over 4% of all CRT candidates are unable to receive lead-based CRT systems due to: unfavourable coronary venous anatomy preventing initial LV lead implant; poor upper limb venous access; or a prohibitively high infection risk.36 In addition, there are theories that leadless LV endocardial pacing may be useful in treating CRT non-responders, where treatment with conventional epicardial lead-based CRT systems is impaired by poor LV lead performance or the presence of myocardial scar.37–39
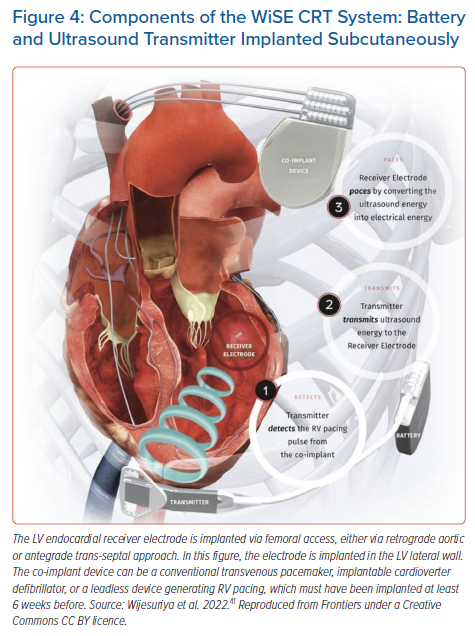
Current RV leadless pacing devices, such as the Micra, have been shown to be too large to be implanted into the LV using computer modelling techniques. Modelling based on cardiac CT images suggests that the Micra would need to be reduced in size by over 40% to be feasible for LV endocardial pacing.40 The only commercially available LV endocardial pacing device is the WiSE-CRT system (EBR Systems).8 This device is commercially available in the European Economic Area and has been given breakthrough device designation by the FDA. The system consists of a battery connected to an ultrasound transmitter, which is implanted subcutaneously at the fourth, fifth or sixth intercostal place, and the receiver electrode, which is implanted in the LV cavity via femoral aortic or trans-septal access (Figure 4).41 The system requires the patient to have a ‘co-implant’ in-situ capable of producing continuous RV pacing, which can be either a conventional transvenous device, or a leadless RV pacing device. The transmitter and battery detect an RV pacing pulse emitted by the co-implant. Within 10 ms of detection of the RV pacing stimulus, the transmitter emits a number of ultrasound pulses to locate the receiver electrode. Once the transmitter is electronically optimally aligned, a longer ultrasound wave is emitted, which is detected and converted to a pacing stimulus by the receiver electrode. This results in LV pacing, and thereby biventricular (BiV) pacing.
The feasibility of using the WiSE-CRT system for delivery of BiV pacing has been tested in observational and registry studies.8,42–45 A meta-analysis of these studies totalling 181 patients reported a procedural success rate of 90.6%, with a clinical response rate of 63% (mean improvement in New York Health Association class of 0.43) and an echocardiographic response rate of 54% (mean improvement in LV ejection fraction of 6.3%).46 It should be noted that the procedure-related complication rate and mortality rate was 23.8% and 2.8%, respectively. The primary life-threatening risk seen was cardiac tamponade, with an incidence of 2.8% (five of 181). Of these five cases, three occurred during the initial WiSE-CRT feasibility study, which was stopped early due to this safety concern.8 The electrode delivery sheath was subsequently redesigned and since then the tamponade rate for this procedure has improved – two out of 167 (1.2%). The most recent observational study of 31 patients reported no pericardial effusions and no procedure-related deaths, suggesting an improved safety profile following a learning curve.43 Results of SOLVE-CRT (NCT02922036), an international multicentre single-arm study of over 150 patients receiving WiSE-CRT are expected in 2023.47
While BiV pacing using an epicardial LV lead is the mainstay of CRT, conduction system pacing (CSP) has become more widespread in recent years. Initially this was in the form of His bundle pacing; however, issues, such as under-sensing and increasing thresholds, may limit its use.48–50 Left bundle branch area pacing (LBBAP) performed by deep penetration of the interventricular septum from an RV approach in an attempt to capture the conduction system has grown increasingly popular, with evidence of good lead performance and heart failure outcomes in early studies.51–57 As such, one area of significant interest is in leadless CSP.
The current generation of leadless RV pacing devices are not designed to provide electrical stimulation to the left bundle system from a right-sided approach. The fixation tines in the Micra system are electrically inert and fan out superficially on deployment, and the outer fixation helix in the Aveir device is similarly electrically inactive.21,58 As such, with the current designs, electrode penetration is not sufficient to reach the left bundle, which runs just under the LV endocardial surface.59 However, LBBAP from the LV aspect has been demonstrated using the WiSE-CRT device.40 The early experience with WiSE-CRT has primarily used a retrograde aortic approach for electrode implantation to target the LV lateral or posterior walls, at an equivalent endocardial position to the location of a conventional epicardial LV lead. However, the development of a trans-septal approach for electrode implantation has given operators the ability to achieve stable positions for deployment on the LV basal septum using deflectable delivery sheaths, such as the FlexCath (Medtronic).60 In a case series, Elliot et al. describe a technique for mapping the septum with a standard decapolar mapping catheter to locate the site of a left bundle potential for electrode deployment.61 Subsequently, further data in this field has been published – histological examination of a porcine model implanted with this technique showed the tines of the WiSE-CRT electrode in close proximity to His-Purkinje tissue, and a case series of eight patients receiving leadless LBBAP demonstrated an improvement in QRS duration with BiV pacing (187.1 ± 33.8 ms versus 149.5 ± 15.7 ms; p=0.009).62 LV-only pacing achieved further QRS reduction (139.8 ± 12.4 ms), suggesting that this may be the best modality moving forward with advancement of the technology – currently, however, the WiSE-CRT system can clinically only provide BiV pacing, with standalone LV pacing only available during device interrogation. The next step in this field would be to perform electroanatomical mapping studies to determine whether CSP is truly achieved by this method as opposed to LV septal myocardial pacing and whether this makes a difference to overall outcomes.
How to Manage Leadless Pacemakers at End of Battery Life
Although the evidence base for leadless pacing is growing, there is no clear consensus on how to manage patients when the devices reach the end of battery life, and this remains an ongoing issue. As the prevalence of leadless devices increases, so will the frequency at which clinicians need to make decisions regarding management in these situations – specifically, whether to retrieve the existing device at the same time as the implantation of the new device. Both human cadaveric and in vivo animal studies have suggested that it is feasible to implant three Micra devices into the RV without device interaction or deterioration in cardiac performance.63,64 While this suggests that retrieval may not be required routinely for end of battery indications, with three devices likely being adequate for the lifespan of many patients with pacing indications, the evidence base for long-term RV performance in the presence of multiple devices is scarce. In an analysis at 24 months follow-up of 989 patients in the Pre-market Micra Transcatheter Pacing Study and the Micra Transcatheter Pacing System Continued Access Study, 11 revisions were required (1.4%).65 There were three successful percutaneous removals and these patients received a new Micra implant. There was one death due to aortic valve endocarditis. In the remaining patients, the Micra device was deactivated; however, all participants subsequently received transvenous pacing. As such, no instances of multiple Micras were observed in this registry.
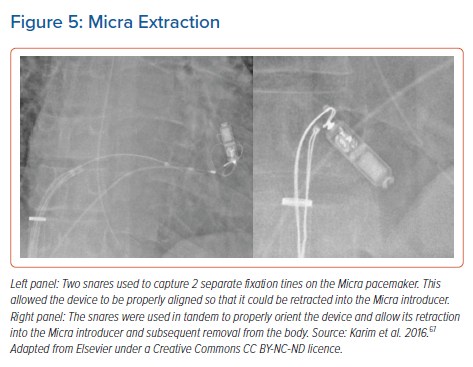
Percutaneous extraction of the Nanostim and Micra devices has been described in observational studies. Dar et al. reported the joint experience of 73 Nanostim and 40 Micra extractions.66 The Nanostim device could be retrieved with a dedicated extraction sheath and these were retrieved with a procedure success rate of 90% at a median time post-implant of 256 days. There is no dedicated retrieval tool for the Micra – extraction is performed via the implant sheath using standard snares to grab both the proximal retrieval pole of the device as well as the distal (RV) portion (Figure 5).67 This can be aided using sheath-in-sheath techniques to introduce smaller steerable sheaths, such as the Agilis NXT model G408319 (Abbott) to aid snaring. There was a 100% reported success rate of Micra extraction using these techniques; however, it must be noted that in this cohort of 40 patients, 20 extractions were performed at less that 24 hours post-implant.66 Further evidence is needed to enable clinicians to balance the risk between extraction and the presence of multiple devices – given the niche nature of this patient cohort and the low frequency of events at short-term follow-up, this data will most likely come in the form of registry studies.
Discussion: the Future of Leadless Pacing
There is certainly growing excitement in the field of leadless pacing. The market is vastly expanding, encouraged by the solid safety and performance profile of the Micra in comparison to transvenous devices. What was initially a field that could only support VVI pacing can now feasibly host a completely leadless CRT-D system by combining leadless RV pacing with a WiSE-CRT device and a subcutaneous implantable cardioverter defibrillator.68 The arrival of leadless atrial devices and the EMPOWER device, which has the capability of providing anti-tachycardia pacing, may further enhance this potential.6,21 Nevertheless, as it stands, leadless systems remain a second-line option for the majority of patients. Their uptake will likely increase gradually with a growing evidence base and education, however, is leadless pacing likely to usurp transvenous pacing soon? Certainly in the realm of VVI pacing this is very possible – we have already seen the trend towards this in the US, and the majority of patients requiring single chamber RV pacing for slow AF, for example, will be of an older demographic and therefore less likely to encounter issues with system revisions and end of battery decisions.
Beyond this, years of further study are needed in more complex pacing modalities. Issues, such as device interaction from different manufacturers, may bring potential problems and having multiple devices whose batteries will reach end of life at different times may expose patients to the need for frequent procedures, despite the adequate battery longevity of each individual device. In addition, the nuanced algorithms which have been developed over decades to improve performance of transvenous pacing are not as mature in the leadless devices and it is unknown how this will affect performance and patient symptoms in the long term. For now, it is exciting that the field is expanding its reach, and it will soon be feasible to provide any form of leadless pacing therapy, whether it be single chamber, dual chamber, CRT or defibrillators, to patients where transvenous pacing is not an option. Gradual expansion of the available data on long-term performance and extraction, as well as technological advancement in device size and battery technology may facilitate these innovative devices moving into the realms of first-line therapy in the years and decades to come.
Clinical Perspective
- For a decade, leadless right ventricular (RV) pacing has been in practice for those patients where conventional transvenous pacing has not been an option.
- Data from Micra-IDE and the Micra Post-Approval Registry has demonstrated a good safety profile of leadless RV pacing compared to transvenous pacing, largely due to the elimination of pocket-related complications.
- While the electrical performance of leadless pacemakers is excellent, atrioventricular synchrony algorithms may under-perform in active patients at high heart rates. Novel devices such as the Aveir atrial leadless pacemaker may address this.
- The WiSE-CRT system has good feasibility data on providing cardiac resynchronisation therapy (CRT) using leadless left ventricular endocardial pacing. This system may also be used to provide conduction system pacing in the future.
- More evidence on device management at end of battery life and extraction will be needed for leadless pacing to be truly considered a viable first-line therapy option.