The use of ICDs for the prevention of sudden cardiac death relies on the ability to deliver a shock to successfully terminate VF. During early adoption of these devices, induction of ventricular fibrillation with defibrillation testing was routinely used at implant to identify the defibrillation threshold. However, it is recognised that defibrillation threshold (DFT) testing is purely probabilistic, meaning that success or failure with a limited number of shocks delivered at a fixed energy does not ensure the same outcome with repeated testing. Together with technological developments and increasing experience with ICD implantation, this has led to the adoption of a programming strategy at maximum device output without DFT testing in most cases. While this empiric strategy may be appropriate for most ICD implants, which are placed in the left pre-pectoral position, specific clinical scenarios may necessitate right-sided implants for which this empiric approach may not be appropriate. The aim of this brief review is to explore the literature behind DFT testing and, specifically, its application to right-sided ICD implants with the aim of informing the development of a shared decision-making tool to assist patients in making fully informed choices alongside their physicians.
Defibrillation Threshold
Although the precise mechanism by which defibrillation results in arrhythmia termination is poorly understood, the basic premise involves the delivery of a voltage gradient through the myocardium, thereby altering cellular transmembrane potentials. The amount of energy required to adequately deliver this voltage gradient and therefore result in defibrillation may be considered the defibrillation threshold. A number of factors influence this threshold, including the nature of the shock waveform, with biphasic waveforms being demonstrated to require lower energy compared with monophasic waveforms.1 Intrinsic factors specific to individual patients, such as myocardial mass or degree of both ventricular hypertrophy or dilatation, are also important and will influence the extent to which the cardiac volume is optimally positioned within the shock vector. In addition, alterations to metabolic conditions and/or electrolyte concentrations, as well as ischaemia or pharmacological interventions, will also impact this threshold. Many of these factors may vary considerably over time, meaning that the precise threshold energy at which defibrillation will be successful can also vary.
A further problem with discussion of defibrillation thresholds is that this figure can never accurately be clinically determined or measured. Although the initial description of defibrillation threshold testing involved the use of repeated VF induction and shock delivery using either escalating or reducing energy levels, even these techniques could only provide a probabilistic threshold above which there was a high chance of successful defibrillation with repeated shock delivery. An infinite number of successful shocks at a given energy level are required to truly identify the point above, which defibrillation is 100% successful, while a single unsuccessful shock may either reflect a point at which defibrillation will never be successful or may, by chance, be the one unsuccessful shock among 99 successful shocks at the same output.
In light of this, and in addition to the wish to avoid a protocol involving repeated VF inductions and defibrillation attempts, most operators have moved to adopt a strategy of safety margin testing, deploying a single shock programmed at an energy level below (often 10–20 J below) the energy delivered by the device. If this is successful, then the additional 10–20 J provides the safety margin in the event of a clinical arrhythmia.2,3
In the earlier years of ICD implantation, DFT testing was often routinely conducted, but the advent of higher-energy devices with optimised waveforms and single coil leads with active ‘can’ configurations, in conjunction with recognition of the risks involved, resulted in increased interest in the need for this approach and conducting important clinical studies.
Current Evidence
Four randomised controlled studies of DFT testing have been conducted. The SIMPLE trial is the largest study evaluating the use of DFT testing at initial implant of ICD devices.4 Healey et al. randomised 1,253 patients to DFT testing and 1,247 patients to undergo no testing using a non-inferiority design and a composite endpoint of failed appropriate shock or arrhythmic death over a mean follow-up of 3.5 years.5 Secondary outcomes included all-cause mortality. The chosen non-inferiority margin was a hazard ratio of 1.5 for the strategy of no testing compared with DFT testing, meaning that non-inferiority would be accepted if the upper bound of the 95% CI was <1.5. The composite primary outcome occurred in 90 (7%) patients in the no DFT testing group and 104 (8%) in the DFT testing group (HR 0·86; 95% CI [0.65–1.14]), giving a p-value for non-inferiority of <0.0001. There were no statistically significant differences in any of the prespecified clinical or safety outcomes. This resulted in the conclusion that omission of DFT testing does not reduce the efficacy of ICD implantation.4
The NORDIC ICD study was a similarly designed randomised, non-inferiority study, but used a clinical endpoint of first shock efficacy for all episodes of ventricular tachycardia (VT) or VF. A total of 540 patients were randomised to receive DFT testing and 537 randomised to not receive a DFT test, with a median follow-up of 22.7 and 22.9 months. All the 218 VT/VF episodes in the patients without DFT testing were terminated with an appropriate ICD shock compared with 96.7% of the 211 VT/VF episodes in the DFT testing group, which was within the pre-specified non-inferiority margin.6 Rates of mortality were not significantly different between groups.6
In addition, two smaller studies were reported.7,8 A substudy of the RAFT trial included 145 patients with 75 randomised to receive DFT testing and 70 to no DFT testing. In only one VT/VF episode was the first shock ineffective, occurring in a patient who received DFT testing.7 A further pilot study was published in abstract form and included a total of 48 patients in whom 12 VT/VF events requiring shock therapy were observed during a mean follow-up of 14.9 ± 7.2 months.8 A systematic review that did not include the results of the NORDIC ICD study, but included patients recruited to non-randomised retrospective and prospective observational studies, also showed no difference in either mortality or the composite of arrhythmic deaths and ineffective shocks between patients undergoing or not undergoing DFT testing.9
These results account for the significant decline in use of DFT testing over the past decade and the change in guidelines to reflect the omission of DFT testing in patients undergoing initial left pre-pectoral transvenous ICD implantation.10,11 However, all these studies excluded patients undergoing right-sided device implants, while the NORDIC ICD study additionally excluded patients with either hypertrophic cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy, making generalisation of these outcomes to these groups difficult. Expert consensus statements reflect this in acknowledging that DFT testing is reasonable in patients undergoing right-sided implants, but do not provide more firm recommendations.11
Current Evidence for Right-sided Implants
Data specifically addressing the use of DFT testing in right-sided implants is lacking. A small retrospective study published in abstract form compared 63 patients who underwent right-sided implants and 1,067 left-sided implants, in whom all received DFT testing. Although the mean DFT was higher in right-sided implants, there was no significant difference in the proportion with high DFTs, defined as >25 J.12 A further study compared DFT in right- and left-sided implants alongside evaluation of the effect of defibrillation waveform tuning.13 Waveform optimisation did not overall affect DFT compared with fixed tilt defibrillation waveform, and the 54 patients randomised to the fixed tilt group were compared with the 54 patients in the fixed tilt group of a parallel trial of matched design in patients receiving left-sided implants.14 The median DFT in left-sided implants was 8.2 J compared with 10.5 J in right-sided implants, while the proportion of patients with DFTs <15 J was 84.6% in left-sided ICDs versus 63.5% in right-sided devices (p=0.014). The proportion of patients with high DFTs (>25 J) was 0% in left- versus 13.5% in right-sided implants (p=0.006).13 However, the comparison between left- and right-sided implants was not a prospectively designed randomised trial and was not large enough to assess meaningful clinical endpoints, including either failed shock therapies or mortality. A further study of intraoperative DFT testing included 39 patients with right-sided implants out of a total cohort of 870 patients, using dual coil ICD leads. The rate of initial DFT >21 J was significantly higher for right-sided versus left-sided implants (p=0.023), but in all patients, revision consisting of lead repositioning, change to single coil lead, reversal of polarity or device repositioning achieved a DFT<21 J.15 Higher-energy output of modern devices, however, will still achieve an adequate safety margin in many of these patients.
Risks of Defibrillation Threshold Testing
An important consideration in performing DFT testing is the risks of the procedure itself. Patients generally excluded from DFT testing include those with known left atrial or left ventricular thrombus, atrial fibrillation without adequate therapeutic anticoagulation, severe aortic stenosis, severe proximal three-vessel coronary artery or left main-stem disease, haemodynamic instability, or recent stroke, as well as the presence of severe respiratory disease precluding safe anaesthesia.16 Major complications generally relate to induction of VF, and include stroke, need for resuscitation and death.16
A meta-analysis that included both the SIMPLE and NORDIC randomised trials, as well as large observational studies, assessed procedural safety using a composite outcome consisting of the sum of complications recorded within 30 days. There was no significant difference in safety outcomes between DFT testing and non-DFT testing cohorts (4.5% versus 2.5%; RR 1.18; 95% CI [0.87–1.60]; p=0.29), largely due to the magnitude of competing risks diluting the low rate of events directly related to VF induction.9 The inclusion of observation studies may also result in inclusion bias, with patients not receiving DFT testing demonstrating greater morbidity. The rate of death within 30 days following DFT test ranged from 0 to 0.9% in the included studies and 0 to 0.6% in those not receiving DFT testing.9
In a large registry study of 64,227 ICD implant procedures performed in 2010, DFT testing was carried out in 71%. Those in whom DFT testing was not performed were older, had higher New York Heart Association function class, lower ejection fraction and were more likely to suffer non-ischaemic cardiomyopathy, as well as have a history of atrial arrhythmias, stroke or chronic lung disease, and were more likely to be receiving cardiac resynchronisation therapy.17 Peri-procedural adverse events were higher (3.58%) in those not receiving DFT testing compared with those receiving DFT testing (2.56%, p<0.001), indicative of the greater morbidity of this group. The mean age in those not receiving DFT testing was 67.4 compared with 62.6 in SIMPLE and 64.7 in NORDIC ICD, as well as 58.8% New York Heart Association III-IV compared with 48.2% (NORDIC ICD; only the New York Heart Association III proportion are reported in SIMPLE, 29.3%), suggesting that these patients may also not be represented in the key randomised studies.4,6,17
Current Practice
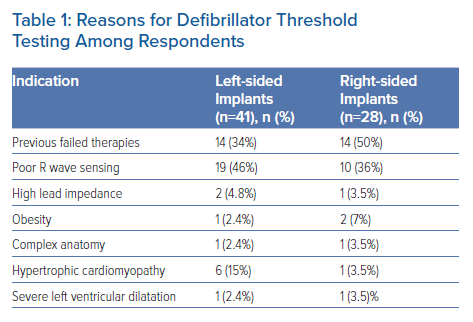
In conjunction with the British Heart Rhythm Society, we conducted a survey of UK cardiologists involved in the implantation of ICDs with the aim of understanding the current use of DFT testing during both left- and right-sided device implants. A total of 105 physicians responded from across the UK. Among those, only 2% routinely perform DFT testing during implant of left-sided devices, although of those who do not do it routinely, 42.6% will do so in specific circumstances (Table 1). In contrast, 27.6% of respondents will routinely perform DFT testing during right-sided device implants, while 41.2% of others will do so in certain circumstances (Table 1). Reasons for considering DFT testing were similar for both left- and right-sided implants, and mainly consisted of sensing concerns or a history of previously failed therapies.
While this highlights that current practices around left-sided devices are in keeping with data from the latest randomised trials, there is a lack of consensus in the context of right-sided implants, with a higher proportion of operators performing DFT testing both routinely and in certain specified circumstances. Given the lack of clinical data on which to base practice, this is not surprising and reflects clinical judgement applied by physicians in interpreting the available data as applied to each individual patient. As in all areas of medicine where clear trial data are lacking and best clinical practice is unclear, clinical decision-making involves informed discussion between the clinician and patients in determining the appropriate approach for that individual and their specific circumstances. This relies on providing the patient with the information needed on which to base their decision in a format that is easily understood, and therefore facilitates a process of shared decision-making between them and their clinician.
Based on the data presented here, and in collaboration with the British Heart Rhythm Society, as well as feedback from patients, we have developed a shared decision-making tool (Supplementary Material Figure 1) to support this process. We envisage that this can be adopted by any clinicians involved in the primary implant of right-sided ICDs to foster an informed discussion with patients in the absence of clearer clinical evidence or specific indications that the physician feels make testing imperative.
Conclusion
While randomised studies evaluating the use of DFT testing have shown no additional benefit or risk compared with a strategy of device implantation without DFT testing, these trials have specifically excluded patients with right-sided device implants. Several small studies have suggested higher DFT in right- compared with left-sided devices, although in the majority, this remains well below an adequate safety margin given the high energy delivered by modern devices and the clinical significance of this finding is therefore unclear. Current evidence suggests that the use of DFT testing is safe, although those patients who may be at highest risk are likely to be underrepresented in the randomised studies. Although modern high-energy devices are likely to achieve an adequate safety margin in the majority of patients with right-sided implants, there is not the same level of evidence to provide the reassurance that exists for left-sided devices. Randomised studies specifically addressing this question are warranted, and until these data are available, a patient-specific approach to DFT testing for right-sided implants is appropriate, considering patient factors that may increase the risk of DFT testing as well as the existence of specific pathologies, including hypertrophic cardiomyopathy or arrhythmogenic cardiomyopathy (groups underrepresented in randomised studies), that may increase the risk of high DFTs. We have developed a shared decision-making tool to support a process of informed discussion between patients and clinicians when considering this difficult clinical problem.
Clinical Perspective
- Defibrillation threshold testing is rarely performed for left-sided ICD implants following the results of high-quality randomised controlled trials.
- Patients with right-sided devices were specifically excluded from these trials, and studies have shown defibrillation thresholds to be higher when devices are implanted on the right side.
- There is very little evidence on the clinical significance of higher defibrillation thresholds with right-sided implants, and use of defibrillation threshold testing during these procedures is highly variable among operators in the UK.
- We encourage a strategy of shared decision-making in this setting and have developed a patient decision aid to support this process.