Pulsed field ablation (PFA) leading to non-thermal irreversible electroporation has been increasingly investigated as an energy source in the field of catheter ablation over the last few years. This review discusses our own clinical findings and current literature focusing on the safety and efficacy of pulmonary vein isolation (PVI) with PFA. Additionally, an overview of PFA targets beyond PVI is provided.
Technology
General Biophysics
PFA offers the unique potential to induce cardiac tissue lesions through the phenomenon of non-thermal irreversible electroporation (IRE). Rapid applications of electrical fields across a cell create small pores in the cell membrane, which ultimately leads to cell death. Contrary to IRE, the effect of reversible electroporation (RE) describes the creation of pores in the cell membrane that seal after a short amount of time, thus not leading to cell death. RE is used to temporarily increase cell membrane permeability for insertion of genes or pharmacological agents into cells. Differences in field strength, number and length of pulses and the distance between the pulses determine whether the electrical fields lead to RE or IRE.1 Application of the electrical field might additionally lead to skeletal muscle activation. A significant difference in skeletal muscle activation between monophasic and biphasic pulsed field waveforms has been observed in a porcine model, with the biphasic waveform not leading to relevant skeletal muscle capture.2
Contrary to ablation with standard thermal energy sources, PFA appears to be tissue-selective, which may allow specific targeting of cardiomyocytes while avoiding injury of surrounding tissue. In AF ablation, tissue selectivity may be particularly important to avoid phrenic nerve and oesophageal injury. This has been suggested by multiple in vivo studies.2,3
In addition, thermal energy sources differ from PFA in the importance of contact force for lesion creation. For radiofrequency ablation (RFA), it has been shown that direct tissue contact with adequate contact force is a requirement for sufficient lesion creation.4 For PFA, an early preclinical animal study demonstrated contiguous transmural lesions in the heavily trabeculated right atrial appendage, suggesting efficient lesion formation even without consistent tissue contact of the catheter.5 A more thorough examination of the relationship between catheter–tissue distance and lesion depth was published recently. The data showed a linear decline of lesion depth with increasing distance, but ablation with a 4 mm distance still resulted in visible myocardial lesions.6 While this proves that PFA – contrary to RFA – creates lesions even without tissue contact, increased contact force with PFA does also increase the lesion size.7 Computational simulations show another important difference between PFA and RFA, whereby blood flow velocity near the catheter does not significantly impact lesion size or morphology in PFA.8
In summary, animal studies and computational simulations suggest that lesion creation with PFA depends less on contact force, catheter orientation and blood flow compared to RFA. However, tissue contact and a certain amount of contact force might still be necessary to create deep lesions.6–8
Ablation Devices
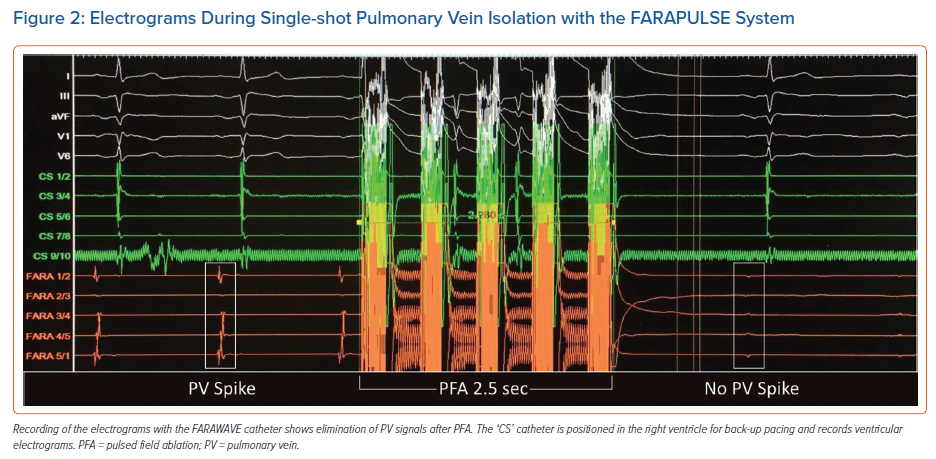
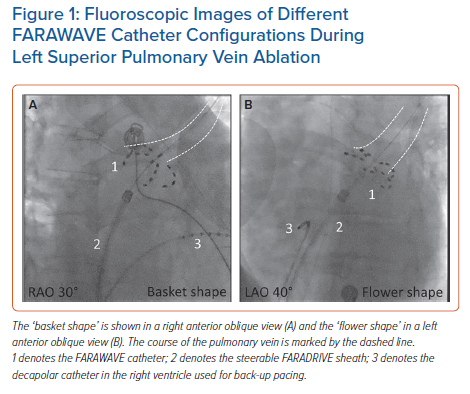
In this review we focus on the FARAPULSE PFA system (Boston Scientific). It is the first device approved for commercial use in Europe. It consists of a generator (FARASTAR) to induce an electric field with an output between 1.8 and 2.0 kV, a 13.8 Fr (inner diameter) steerable sheath (FARADRIVE) and a 12 Fr over-the-wire catheter (FARAWAVE). The catheter has five splines with four electrodes per spline and its configuration can be changed seamlessly between a ‘flower-like’ and a ‘basket-like’ pose (Figure 1). It is available in two different sizes (31 mm and 35 mm).9 The third electrode of each spline allows for recording of electrograms and pacing (Figure 2). The catheter is designed for single-shot isolation of the pulmonary veins (PVs), comparable to a cryoballoon or a radiofrequency balloon. The success rates of single-shot isolation has been shown to be less operator-dependent compared with point-by-point RFA for PVI.10
The Galaxy Centauri system (Galvanize Therapeutics) follows a different approach. Its generator enables conventional RFA catheters to deliver PFA, thus allowing for focal tip PFA. The system is compatible with three different ablation catheters from three major manufacturers, therefore allowing electrophysiologists to deliver PFA through catheters they are already accustomed to. Studies focusing on the safety and efficacy of this system are currently enrolling patients or have recently finished enrolment (NCT04523545).
The VARIPULSE catheter (Biosense Webster) is a circular catheter with an adjustable size that is designed to deliver PFA at the PV ostia. Results of the inspIRE study gathering data on its safety and efficacy are expected soon (NCT04524364).
The Affera system (Medtronic) provides a hybrid RFA/PFA catheter. The 9-mm lattice-tip catheter can be toggled between delivering PFA and RFA and can be visualised in an electroanatomical mapping system. Data on successful PVI, as well as left atrial linear ablation, using this system have already been published.11
Pulsed Field Ablation in Atrial Fibrillation
Procedural Strategy
In the first studies, general anaesthesia with neuromuscular blocking agents was a necessity because of the use of monophasic PFA.12 Skeletal muscle activation would have otherwise led to extensive motion of the patients. The switch to biphasic PFA led to the possibility of procedures under conscious sedation without neuromuscular blocking agents. Initial studies were designed to allow continuous changes of the ablation settings to determine the optimal pulsed field waveform protocols.9
Following the optimisation of the waveform, the 5S study (Safe and Simple Single Shot pulmonary vein isolation with pulsed field ablation using Sedation) focused on streamlining of the procedure. All procedures were performed with a single transseptal sheath. Additionally, one decapolar diagnostic catheter was placed in the coronary sinus for the transseptal puncture and was repositioned to the right ventricle during the ablation for backup pacing in case of bradycardia. During the first 25 procedures (validation phase), a circular mapping catheter was inserted to confirm PVI through acquisition of PV electrograms before and after the ablation. Interestingly, this always confirmed the mapping findings of the FARAWAVE catheter. Therefore, the catheter exchanges and re-mapping using the standard circular mapping catheter was omitted from the workflow in the following 166 procedures (streamline phase). All procedures were performed under deep sedation without general anaesthesia. During the initial validation phase, the procedure time was 46 ± 14 minutes. This decreased to 38 ± 14 minutes during the streamlining phase.13
Acute Procedural Data
Feasibility of PVI using the FARAPULSE ablation system in paroxysmal AF was shown in 2019 in the two first-in-human trials: IMPULSE and PEFCAT.9 The procedural endpoint was defined as PVI. In a total of 81 patients, all PVs were successfully isolated using PFA with a mean procedure time of 92.2 ± 27.4 minutes including a 20-minute waiting period. Study protocols mandated electroanatomical voltage mapping of the left atrium and the PVs during the index procedure after PVI. In 2021, Reddy et al. published additional data on PFA in paroxysmal AF, now including the PEFCAT II trial added to IMPULSE and PEFCAT. In a total of 121 patients, acute PVI was achieved in 100% of patients.14
Following these initial studies of the role of PFA in paroxysmal AF, the use of the technology was extended to persistent AF. The PersAFOne study included 25 patients with a median age of 67 years (IQR 60–70 years). Acute PVI was successfully obtained in 100% of patients. Importantly, the ablation strategy was extended by successful left atrial posterior wall isolation, which will be discussed in a later section of this review. The additional ablation accounted for a longer procedure time of 125 minutes (IQR 108–166 minutes), including a waiting period of 20 minutes.15
The 5S study was investigated in a large single-centre cohort. PVI with PFA was performed in a total of 191 patients. Baseline characteristics represented a typical all-comer AF collective, with 58% male patients, mean age 69 ± 12 years and mean BMI 28 ± 5 kg/m2. Patients with paroxysmal AF (64%), as well as persistent AF (36%), were treated with PFA. All PVs were successfully isolated using PFA with a high rate of isolation during the first application (99.5%).13
The recently published MANIFEST-PF survey among 24 centres in Europe summarises the experience of PFA in a total of 1,758 patients. This includes patients from the above-mentioned studies. Patients with paroxysmal AF (57.5%) and persistent AF (39.1%) with a mean age of 61.6 years were included. The acute success rate of PVI was 99.9%, with a mean procedure time of 65 minutes. The procedure was performed without general anaesthesia/intubation in 82.1% of patients.16
Safety
The MANIFEST-PF survey provides an overview of complication rates among a large patient cohort. Major complications (pericardial tamponade, stroke, vascular injuries requiring surgical management) occurred in 1.6% and minor complications (mainly vascular injuries with conservative management, transient phrenic nerve injury and transient ischaemic attack) in 3.3% of the patients. Stroke occurred in seven patients (0.39%) and one stroke led to the only death (0.06%) in this patient population. In at least three of these cases, the operators attributed the stroke to catheter or sheath exchanges.16 They might be related to the initial learning phase and possibly improper handling of the large 13.8 Fr sheath.13 In addition to these symptomatic events, brain MRI showed silent cerebral lesions in one of 18 patients from the IMPULSE, PEFCAT, PEFCAT II study and in 10 of 53 patients in the 5S study.14,13 These findings are similar to reported incidences of silent cerebral lesions in AF ablation with contemporary thermal ablation technologies.17 It is of note that these data include the complete learning curve of the operators. A continuous decrease in complication rates with increase in experience could be observed in MANIFEST-PF when the timeline of reported cases was divided into tertiles for each participating centre.16
Cerebral events, vascular complications (3.41% in MANIFEST-PF) and pericardial tamponade (0.97%) are non-thermal complications and are neither specific for thermal ablation nor for PFA. On the other hand, PV stenosis, oesophageal lesions and phrenic nerve palsy can be linked to thermal ablation. This review will therefore provide more detail on these complications in the following sections.
Pulmonary Vein Stenosis
Thermal and distal PV ablation can lead to loss of collagen matrix and thereby may cause PV stenosis.18 In contrast, the novel PFA system using non-thermal IRE was not associated with PV stenosis in animal studies and initial clinical experience.5,14,19,20
Oesophageal Lesions
Tissue selectivity sparing the oesophagus was suggested in preclinical animal studies and was confirmed in all reviewed clinical studies. In IMPULSE, PEFCAT, PEFCAT II and the majority of 5S patients, no oesophageal temperature monitoring was used. In a subset of 90 patients of these studies oesophagogastroduodenoscopy (OGD) was performed after the ablation and ruled out oesophageal lesions.13–15 Furthermore, MRI was performed in 18 patients to evaluate the ablation lesions in more detail confirming no gadolinium enhancement in the oesophageal tissue. Of note, even adjunctive posterior wall isolation (PersAFone) did not lead to OGD-detected oesophageal lesions.15
Phrenic Nerve Palsy
IMPULSE, PEFCAT, PEFCAT II and PersAFOne followed a protocol of phrenic nerve assessment which mandated thoracic fluoroscopy at the end of the procedure as well as 3 months after the procedure. In a combined 146 patients, no phrenic nerve palsy was observed at the end of the procedure. Overall, 126 patients underwent additional chest fluoroscopy after 3 months and again showed no signs of phrenic nerve palsy. The authors reported frequent phrenic nerve capture during PFA, especially during ablation of the right superior pulmonary vein.14,15
The amount of phrenic nerve capture was quantified in the 5S study. Contractions of the diaphragm were observed in 166 of 191 ablations of the right-sided PVs and 118 of 191 ablations of the left-sided PVs. In two patients, a transient palsy of the right-sided phrenic nerve was observed, which recovered in <1 minute. Both patients were re-evaluated using fluoroscopy the next day and showed no signs of sustained phrenic nerve palsy.13,21 This effect of transient phrenic nerve palsy was also reported in a total of eight patients (0.46%) in the MANIFEST-PF survey. No sustained palsy was observed.16 Such temporary stunning of the phrenic nerve without sustained injury was also observed in an animal study. While transient weakening and even loss of phrenic nerve function could be induced through PFA, these effects resolved completely during the procedures. Interestingly, histopathologic evaluation of the nerves showed no signs of injuries, even in the animals treated with the highest PFA voltage levels in close proximity to the phrenic nerves.22
Coronary Vasospasm
PFA directly on coronary arteries can induce vasospasm in animal models.23,24 Of note, Gunawardene et al. reported a case of coronary vasospasm of the ramus circumflexus after PFA of the mitral isthmus in close proximity to the vessel. Intracoronary nitroglycerin completely reverted the vasospasm.25 Lemoine et al. described transient ST-elevation and complete heart block for 3 minutes during one of the PFA procedures.26 No coronary angiography was performed, and the ECG changes resolved spontaneously.
Recently, Reddy et al. performed coronary angiographies before and after PFA in 25 patients. They were able to show that PFA near coronary arteries does frequently induce vasospasm and can be prevented by nitroglycerin treatment. Neither PVI nor posterior wall isolation-induced coronary vasospasm. However, patients receiving PFA of the cavotricuspid isthmus (CTI) showed severe spasm of the right coronary artery. This was completely avoided if intracoronary or intravenous nitroglycerin was administered before ablation.27 These data raise the question of whether parenteral nitroglycerin application before ablation should become the standard of care for PFA in proximity to coronary vessels.
Outcome
Due to the very recent introduction of the FARAPULSE PVI system, large-scale mid-term and long-term outcome data remains scarce.
The IMPULSE, PEFCAT and PEFCAT II study design included systematic remapping procedures to assess PVI durability. Of the 121 patients, 110 underwent remapping 93 ± 30.1 days after the index procedure. While PV remapping initially showed durable PVI in only 18.1% of patients and 45.2% of PVs, this was increased to 84.1% of patients and 96.0% of PVs through waveform protocol optimisation. Most reconnections were found in the superior PVs.14 This finding was confirmed in other studies and a more detailed analysis showed that the PV size may play a significant role, with larger PVs showing more reconnections and the superior PVs being commonly larger in diameter than their inferior counterparts.28,29 Since both the 31-mm and 35-mm FARAWAVE catheters usually cover the whole PV-ostium, the reasons for more reconnections of larger-diameter PVs are not obvious and need further research. The density of the electric field at different distances from the catheter might play a role.
The 5S study protocol did not include remapping procedures, but recently published data on procedures for recurrent atrial arrhythmias in these patients provides insights into PV durability. Of 360 patients who were treated with PFA according to the 5S protocol, 46 patients developed recurrent atrial arrhythmia and 25 of these underwent a second ablation procedure. Durable PVI was observed in 76.0% of patients and 90.9% of PVs. Left superior PVs showed the highest number of reconnections.28 These results can of course not be compared to the remapping results of IMPULSE, PEFCAT and PEFCAT II, as only patients with recurrent atrial arrhythmias underwent the procedures. In these patients, a higher number of PV reconnections is to be expected compared to patients without recurrence of arrhythmias. Furthermore, these remapping procedures showed atrial tachycardia (AT) instead of recurrence of AF in 64% of the patients. The mechanism of AT was related to a critical isthmus at the left atrial posterior wall between the PV lesions in 61.5% of all AT patients. This unusual high incidence of AT and, in particular, left posterior wall dependent AT might be attributed to extensive lesion formation around the PVs, which creates a narrow channel of slow conduction between the lesions.
Reddy et al. reported the 1-year follow-up data of patients with paroxysmal AF. The Kaplan–Meier estimate for freedom from any atrial arrhythmia at 1 year was 78.5 ± 3.8%. This included the initial patients who were not treated with the optimised waveform protocol and showed a high number of PV reconnections. Considering only the patients treated with the optimised waveform, the respective Kaplan–Meier estimate went up to 84.6 ± 6.0%. None of the patients treated with the optimised waveform protocol who experienced recurrence of atrial arrhythmia showed PV reconnections. One year after the procedure, Class I and III antiarrhythmic drugs were used in 13% of the optimised waveform cohort.14
Lemoine et al. reported on the long-term outcome of 138 patients treated with PFA at two German high-volume centres. Mean age was 67 ± 12 years and a high number of patients with persistent AF (62%) were included. PVI after the first application of PFA was achieved in 99.6% of pulmonary veins. The 1-year follow-up showed Kaplan–Meier estimates for freedom from any atrial arrhythmia of 90% ± 6% in paroxysmal AF and 60% ± 10% in persistent AF. At the last follow-up visit, Class I and III antiarrhythmic drug therapy was continued in only 2.4% and 2.1% in patients with persistent and paroxysmal AF, respectively.26
Beyond Pulmonary Vein Isolation
The efficacy of left atrial posterior wall (LAPW) ablation was systematically investigated by Reddy et al. in the persistent AF collective. They performed LAPW ablation in all 25 patients and found durable lesions at the posterior wall in 18 of 21 patients undergoing the remapping procedure. Additional ablation of the CTI was performed in 13 patients and showed durable block during remapping in nine of 12 patients. However, this ablation was performed using a different catheter to apply focal lesions and the ablation protocol was optimised during the course of the study, increasing durability of CTI block from 25% before the optimisation (1 of 4) to 100% after the optimisation (8 of 8).15
Lemoine et al. reported successful CTI ablation in four patients and LAPW ablation in one patient. Additionally, two patients received PFA ablation of the left atrial anterior wall due to AT, which terminated the tachycardia. In one patient, AF triggers from the superior vena cava (SVC) were identified and PFA in the SVC was performed. Afterwards, an episode of sinus arrest was observed (5 minutes) but resolved completely. Currently, there are no published data on remapping procedures from this collective.26
A recent publication evaluates the feasibility of PFA for left atrial tachycardia (LAT) after previous AF ablation. Gunawardene et al. used PFA to successfully terminate 18 macro-reentry LAT and one localised-reentry LAT. They furthermore performed ultra-high-density mapping after the ablation and proved successful block of all ablation lines (13 roof lines, 11 anterior lines and one mitral isthmus line).30
Case reports of additional rare AF and AT ablation targets have been published. Successful isolation of a persistent left SVC was performed in two patients.31 One case of successful termination of an AT from the right atrial appendage by PFA after failed RFA has been published.32
Knowledge Gaps
No randomised controlled trial (RCT) comparing the long-term results of PFA with one of the already established thermal energy sources has been published to date. Data on the high durability of PVI seems to be sufficient. But the question of whether this translates to at least the same or even lower rate of recurrence of atrial arrhythmias compared to other energy sources remains open. This is especially of interest because PFA shows a significantly lower effect on the autonomic cardiac nervous system than RFA, most likely due to the tissue selectivity, which spares the ganglionated plexi.33 The resulting clinical effect has been researched through a comparison of heart rates 3 months after ablation. While a significant increase of the heart rate could be observed after thermal ablation, PFA showed no such effect, suggesting no relevant impact on the autonomous nervous system.34 However, the implication of this effect on long-term freedom from arrhythmia is not yet well understood and can only be answered adequately by an RCT comparing PFA with thermal ablation. The ADVENT trial might be able to answer to these questions in the future. It is an on-going multi-centre RCT comparing PFA to thermal ablation and has recently finished enrolment.
While patients with paroxysmal, persistent and long-standing persistent AF were treated with PFA, outcome data of patients with long-standing persistent AF are sparse.16 However, it has to be considered that the correct ablation strategy in this patient collective is still under debate. Whether PFA is seen as a suitable energy source in these patients currently depends on the desired lesion set.
Concerning the safety of PFA, further scientific attention should be focussed on the details and mechanisms of coronary vasospasm. While the trials already conducted lead to little concern regarding PVI, the use of PFA has already been extended to atrial extra-PV targets and to the ventricles for ablation of premature ventricular contractions.35 This requires a close evaluation of possibly dangerous coronary vasospasms.
PFA in patients with cardiac implantable electronic devices (CIED) has only been reported in small series and case reports.36,37 While these showed no negative effects, larger case series are needed to definitely rule out any short-term or long-term damage of CIEDs due to PFA.
The extensive PFA lesions around the PVs may create narrow channels on the left atrial posterior wall, thus providing an isthmus for roof dependent AT.28 This possibly proarrhythmogenic effect should be investigated further. Knowledge of this effect might influence the choice of the catheter size in PFA with FARAPULSE. Avoiding creation of a relevant isthmus on the posterior wall may be achieved through ablation with the smaller 31 mm catheter. Alternatively, routine posterior wall isolation during the index procedure could be pursued in patients at risk for development of roof dependent AT after PFA.
Perspective
This review attempts to summarise the published data on using the FARAPULSE ablation system for AF ablation. The reviewed data show that PFA is a safe and effective method for PVI and results in a high percentage of durably isolated PVs. It also shows feasibility for additional ablation targets beyond PVI. Tissue selectivity of non-thermal PFA and its positive safety effects during ablation in the left atrium was confirmed.
The results of the IMPULSE, PEFCAT, PEFCAT II, PersAFOne and 5S trials show the fast evolution from first-in-human trials and optimisation of the ablation settings to further streamlining of the procedure. Optimisation of the waveform protocols led to high durability of the applied lesions while procedure times were gradually reduced through the decrease of intraprocedural pre- and post-ablation mapping. The speed and safety of the procedure may lead to PFA being preferable for patients with multiple comorbidities, in which short procedure times and use of conscious sedation instead of general anaesthesia are particularly important.37 Furthermore, short procedure times might provide the potential for a higher cost-effectiveness of PFA compared to other ablation modalities. However, this comparison is complex and should take cost of the device itself into account. Furthermore, different healthcare systems around the world must be considered when comparing cost-effectiveness of interventions.
The results of on-going clinical trials examining the use of the FARAPULSE, Affera and VARIPULSE systems in the US are expected soon and will certainly provide more insight on safety and efficacy of PFA for PVI. Development and distribution of these different PFA systems integrating 3D mapping and focal ablation will also further extend the use of the technology. Focal ablation allows for complex lesion sets and the high lesion durability of PFA may be used to further investigate ablation targets other than the PVs in persistent AF. Looking beyond AF, PFA might also have an impact on ablation of ventricular tachycardia. Due to the diameter of the left ventricular myocardium as well as the trabeculated endocardial surface, the application of lesions in the left ventricle is difficult. PFA as a new technology may help to achieve durable transmural lesions.
Clinical Perspective
- Pulmonary vein isolation using non-thermal pulsed field ablation with the FARAPULSE ablation system is safe and efficient.
- Pulsed field ablation shows cardiac tissue selectivity in a clinical setting, reducing the risk of oesophageal injuries, pulmonary vein stenosis and phrenic nerve palsy.
- Durability of pulmonary vein isolation with pulsed field ablation is high.
- No final statement regarding the long-term clinical efficacy of pulsed field ablation compared to thermal energy sources can be made yet because of a lack of randomised controlled trials.