AF is the most common sustained arrhythmia, both clinically and worldwide, with a lifetime incidence estimated at ~20%.1,2 The total number of cases of AF in the US is expected to exceed 2.5 million by the year 2030 and 7.5 million by 2050.3,4 Furthermore, AF carries significant morbidity and is one of the leading causes for hospitalisation in the US, with annual AF admissions exceeding 450,000 cases per year since 2010.3,4
In developed countries, common neurological diseases – including dementia – are increasingly prevalent and account for a significant burden on society.5 Population longevity and ageing are leading contributors to this trend.6 These adverse associations are compounded in patients with AF, which drives morbidity and mortality in patients with neurological diseases.7 Recent studies have begun to explore the concept of cerebrovascular reserve and risk of idiopathic dementia, such as Alzheimer’s disease.8 This concept of microvascular disease as a chronic cause of cerebral vascular hypoperfusion begins to link the brain more broadly to vascular health. Similarly, AF is also a manifestation of both macro- and micro-vessel disease that result in elevated filling pressures, left atrial enlargement and atrial arrhythmias.
As a consequence of patients living longer following AF diagnosis, the detrimental impact of this arrhythmia and its treatments can be seen in long-term end organ dysfunction, including cerebral pathology. AF has a clinical association with a broad spectrum of central nervous system pathology, including clinical and subclinical strokes, clinical and subclinical bleeds, repetitive microbleeds, cerebral hypotension/hypoperfusion, atrophy and volume loss, grey matter changes and amyloid plaques.9 It is critical to identify the underlying mechanisms of these associations and continue to explore focused treatment approaches for AF and its comorbidities that may benefit cerebral function chronically.
AF and Dementia
The association between AF and dementia is multifactorial and can present both in the acute setting following a stroke and in the chronic setting due to progressive cognitive impairment or multiple repeated injuries. Acutely, AF can precipitate stroke in the setting of the arrhythmia and conversion to normal sinus rhythm. This has been traditionally the most feared complication of AF, as these strokes are associated with significant morbidity and mortality.10,11 However, it is not uncommon in clinical practice for patients to describe cognitive dysfunction or ‘brain fog’ as a symptom of AF. These symptoms tend to reverse or improve with restoration of sinus rhythm. One of the limitations of historic research in AF has been the general application of symptoms to all patients with AF. Elderly patients at risk for cognitive decline and dementia may not report concerns of dyspnoea or palpitations when climbing stairs, but instead report difficulty in concentrating or processing when in AF. In a study from the Utah My Evaluation programme, younger patients were much more likely to correctly identify their AF and associate them with traditional cardiac symptoms.12 We have also recently found that symptoms of middle- and late-onset depression in patients with AF may be an early sign of dementia and also contribute to disease mismanagement that can increase dementia risk.13
Multiple possible mechanistic pathways have been proposed to explain this spectrum of acute and chronic brain injury manifestations, such as micro- and macro-embolic events, micro- and macro-haemorrhagic events, haemodynamic changes related to perfusion and/or pulsatility, atrioventricular asynchrony causing arterial hypotension and capillary hypertension, poor cerebrovascular reserve, and extra- and intra-cranial vascular disease that impacts adaptation, among others.9,14–18
One pathway of particular interest involves embolic and haemorrhagic states that are associated with AF. Classically, AF has been associated with micro and macro-embolic events felt to originate from left atrial thrombus formation in the setting of AF. The timing and development of these ischaemic events and the underlying mechanisms remain controversial; in part due to the lack of consistent temporal association between arrhythmia onset and stroke events.19–21 Nonetheless, the mainstay of AF treatment remains chronic anticoagulation to lower the risk of ischaemic stroke, although this therapy also can cause micro and macro-haemorrhagic events. These thrombotic and haemorrhagic events do not always present acutely or in a clinical manner, but instead they may lead to progressive cognitive impairment (subclinical and clinical), cerebral dysfunction and volume loss over time.9 In this pathway, the timing, use, agent selection and efficacy of anticoagulation are critical.
Brain ischaemic injury, whether clinical or subclinical, does not account for all the increased risk of cognitive decline and dementia in patients with AF.22 Nor can it account for the abrupt cognitive symptoms many experience with AF onset. An alternative pathway relates to the haemodynamics during AF when compared to haemodynamic measurements in normal sinus rhythm and may explain the abrupt onset of cognitive decline that seems immediately reversible with reversion to sinus rhythm. A study performed by Saglietto et al. showed that haemodynamic stability and hypoperfusion episodes are often described in AF.18 This vascular dysfunction reflects varied R-R intervals and atrioventricular asynchrony causing repetitive hypoperfusion at the arteriolar level and hypertension at the capillary level.
This vascular dysfunction reflects varied R-R intervals and atrioventricular asynchrony leading to repetitive hypoperfusion at the arteriolar level and hypertension at the capillary level. Cerebral perfusion imaging of patients with AF identify perfusion deficits globally compared to sinus rhythm, but in particular the inferotemporal regions that are critical in memory and recall.18 Cerebral hypoperfusion has been shown to be associated with the presence and severity of white matter lesions and as these lesions increase or progress cognitive dysfunction risk increases.23
As the risk factors for vascular disease increase, vascular disease is diagnosed, or systemic health worsens, both men and women with AF have increased risk of dementia.24 Patients with carotid arterial disease have an increased risk of stroke and dementia and have been reported to be the most likely to benefit with an aggressive rhythm control approach to lower these risks compared to those patients without carotid arterial disease.25
Restoring Sinus Rhythm to Lower Stroke Risk in Patients with AF
If AF is associated with stroke risk, then it seems plausible that treatment of the abnormal rhythm would lower stroke rates. However, randomised controlled trials of rhythm versus rate control (AFFIRM and AF-CHF) failed to show a reduction in stroke risk in those treated with antiarrhythmic drug therapy.26,27 The lack of benefit was explained in part by discontinuation of anticoagulation, which occurred more often in the rhythm control group, as well as lack of efficacy of antiarrhythmic drug therapy. The latter explanation was supported by a subanalysis of the AFFIRM trial that found that patients who maintained sinus rhythm had lower stroke rates compared to those who did not.28
Recently, the EAST-AFNET 4 trial, published in 2020, randomised 2,789 patients within 1 year of AF diagnosis to either early rhythm control or standard treatments prioritizing rate control. Differing from historical studies, this study demonstrated a lower risk of adverse cardiovascular outcomes in the early rhythm control arm, including stroke.29 Compared to AFFIRM, EAST-AFNET 4 only included patients with recently diagnosed AF (<12 months) and a high adherence to anticoagulation in both arms of >90% throughout the study follow-up duration.
Similarly, the ATHENA trial randomised 4,628 participants to receive either the antiarrhythmic drug dronedarone or a placebo.30 This study demonstrated reduced cardiovascular hospitalisations and deaths in AF patients who were successfully rhythm controlled. The ATHENA trial included patients that were relatively healthy – or earlier in the AF disease history – with a mean CHADS2 score of 2. In a subanalysis of this study, dronedarone reduced the risk of stroke from 1.8% per year to 1.2% per year (HR 0.66; p=0.027).31
Taken together, these studies strongly support the use of medical rhythm control, on a background of strong adjunctive medical therapy (e.g. anticoagulation and blood pressure control) to improve neurological outcomes among patients with AF.
Catheter Ablation of AF to Lower Stroke Risk
Large population-based observational trials have found that AF ablation is associated with lower long-term stroke rates and mortality compared to medical therapy for AF alone. In a study of the Intermountain Healthcare registry, 4,212 consecutive patients who underwent their first AF ablation and had at least 3 years of follow-up and compared them 1:4 to 16,848 age- and sex- matched controls with AF (no ablation) and 16,848 age- and sex- matched controls without AF.32 In patients with AF with no ablation, there was a significant increased risk of stroke/cerebrovascular accident at 1- and 3-year follow-up (OR 2.12; p<0.0001 and OR 1.90; p<0.0001, respectively) compared to those patients with AF who underwent an ablation. In patients with AF who underwent an ablation there were no significant differences at 1- and 3-year follow-up (OR 0.94; p=0.68 and OR 0.86; p=0.21, respectively) compared to patients without AF. In those AF ablation patients in this registry without any observed recurrence of AF, the stroke rates were very low at 1.0% at 1 year and 1.7% at 3 years in a population with an average age of 65 years in which 6% had a prior stroke, 45% had hypertension, 21% had diabetes and 24% had heart failure. In this study, anticoagulation use was strongly advocated over the first year after ablation, but information regarding long-term use was not available.
Using the same database, outcomes were assessed in high-risk patients with AF for recurrent stroke that had a prior history of stroke. The 5-year risk of stroke (HR 2.26; p<0.0001) and death (HR 2.43; p<0.0001) were higher in the AF with no ablation group compared to those that were ablated. AF ablation patients compared to no AF patients experienced similar 5-year risks of stroke (HR 0.82; p=0.39) and death (HR 0.92; p=0.70) despite higher heart failure risk (HR 3.08; p=0.001).33
Other large observational studies have demonstrated similar findings. In a study of 361,913 patients with a diagnosis of AF in the Swedish Patient registry, catheter ablation was associated with lower risk of stroke (HR 0.69) and mortality (HR 0.50) compared to patients with AF that did not receive an ablation.34 In an institutional propensity-score-matched study of 3,953 (83.4% patients with a CHA2DS2-VASc score of ≥2), a reduction in stroke/transient ischaemic attack rate was observed in the ablation group compared to a non-ablated group (HR 0.61) and remained so after adjustment for the baseline CHA2DS2-VASc score.35 In a multicentre registry of 1,273 patients, freedom from AF was associated with stroke-free survival (HR 0.30). Also, the rates of stroke and death were significantly lower in this registry cohort (0.5%/patient-year each) compared with AF patient treated medically in the Euro Heart Survey (2.8% and 5.3%, respectively; p<0.0001).36
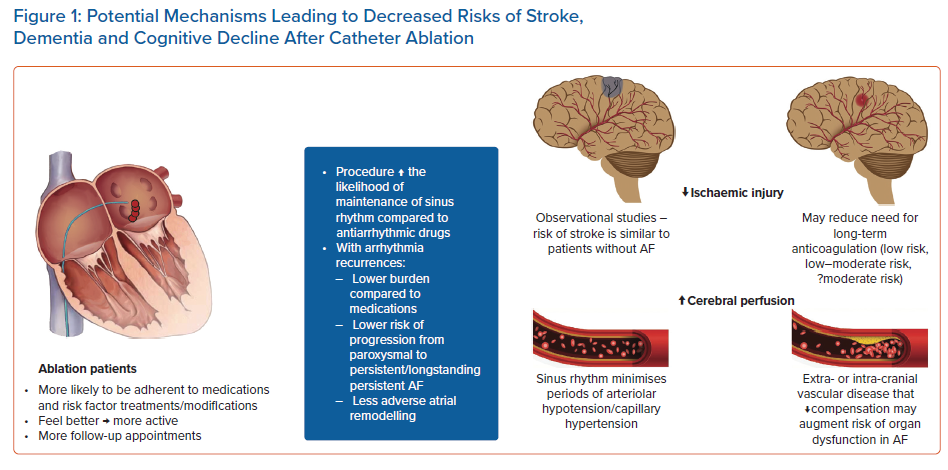
These studies strongly suggest interventional approaches to rhythm control (e.g. catheter ablation) are associated with improved neurological outcomes compared with medical therapy, despite a short-term increased risk of stroke associated with such procedures.
Restoring Sinus Rhythm to Lower Risk of Cognitive Decline and Dementia in Patients with AF
Several studies have examined the impact of rhythm versus rate control on metrics of brain perfusion and/or cognition.37–40 In a study of 44 patients with AF who underwent cardioversion, restoration of sinus rhythm improved brain perfusion and cerebral blood flow.40 In another study that examined the role of rhythm and cerebral perfusion, perfusion was less in patients in AF compared to sinus rhythm, but also worse in those with persistent AF compared to paroxysmal.39 In a retrospective analysis of 272 patients that compared a rhythm control strategy versus rate control with anticoagulation, a rhythm-control strategy was associated with a lower risk of cognitive impairment as measured by cognitive performance testing.38
Personalised medicine for patients with AF may identify risk of cognitive decline and dementia beyond traditional risk scores, which provide relatively poor predictive accuracy.24 This idea of more personalised treatment plans for AF in the setting of cognitive function is explained by the multiple variabilities between patients, including drug–drug interactions, ageing, genetics and medicine adherence. Controlling these risk-related comorbidities and understanding their severity is dynamic and should ideally be tailored in each setting to lower risk of cognitive decline. For example, using common lab measurements of health, such as the complete blood count and basic metabolic profile, an augmented intelligence tool can stratify risk of dementia in both men and women in addition to that assigned historically by the CHA2DS2-VASc scoring system into low, medium and high risk.24 Consideration of this tool used here as an example, and others that may emerge, is important as we explore therapeutics which may alter risk – something that cannot be accomplished with static or regression scores, such as CHA2DS2-VASc.
Catheter Ablation of AF To Lower Risk of Cognitive Impairment/Dementia
Catheter ablation is a more efficacious and durable approach to AF compared to antiarrhythmic drugs.41 Recent trial evidence supports its use early in the disease course of AF to improve long-term outcomes and lower risk of arrhythmic recurrences and AF burden.42,43 Catheter ablation, in comparison to antiarrhythmic medications, is a more effective modality and may reduce the risk of brain dysfunction/cognitive impairment in the following studies described below. There are several mechanistic pathways in which ablation may improve or preserve brain health (Figure 1).
Multiple large-volume epidemiological studies have shown benefit on brain function with AF ablation. Using the Korean National Health Insurance database, a 2020 nationwide cohort study identified 194,928 adults with AF treated with either catheter ablation or pharmacotherapy either using antiarrhythmic or rate-control drugs. They selected 9,119 patients undergoing ablation and 17,978 patients managed with medical therapy to follow for a median course of 52 months. When compared to the medical therapy group, those patients treated with catheter ablation showed lower risks and overall incidence of dementia (8.1 versus 5.6 per 1,000 person/years).44 This benefit persisted with censoring for prevalent and incident stroke.
In the previously described population-based study of 4,212 AF patients treated with ablation matched 1:4:4 with patients with a history of AF who did not receive a catheter ablation and population without AF, long-term general and Alzheimer’s dementia rates were similar in the patients with AF who underwent ablation and those patients without AF, both of which were better than patients with AF not treated with ablation.32

Data from the large Swedish health database further support these outcomes after ablation. Friberg et al. identified 5,176 ablations performed across 4,728 patients over a 7-year period.34 When compared with AF patients managed without catheter ablation, those receiving ablation had lower incidents of stroke, transient ischaemic attack and death.
Other large trials have largely supported these findings (Table 1), including those that have included more specific cognitive testing. Of these trials, one used serial cognitive testing in 308 patients who underwent ablation compared to 50 patients with AF who did not.45 In this trial, the Montreal Cognitive Assessment (MOCA) score improved in patients who underwent ablation and this improvement was seen when comparing different age groups and AF severity subtypes. Of particular interest, the area of most improvement in the MOCA was in memory/recall, a critical finding when considering the observational studies that report dementia rates are reduced in patients that undergo an ablation.
It should be noted that there is controversy about the mechanisms of ablation impacting dementia risk and if there is true benefit.46,47 This controversy originates from several factors, including selection bias whereby healthier patients are referred for ablation, improved clinical management of comorbidities in patients who receive AF ablation and the recognition that ablated patients receive more frequent follow-up. This possibility is supported in part by a discrepancy in data regarding a reduction in stroke after ablation between randomised control trials and observational trials.46 In randomised control trials outcomes are similar in regard to stroke compared to discrete differences in observational trials in which ablation patients experience much lower stroke rates. Some of this reflects a randomised trial design in which patients must be selected for the trial and meet an often relatively narrow inclusion criterion. Next, a recent study of 30 patients with AF did not find a preferential benefit in cognitive function in patients with a low AF burden compared to a high burden.47 Given the data we have presented, it would seem that patients who experience a lower AF burden should also have improved cognition. However, these outcomes were from a relatively short follow-up period of 6 months, a low number of patients were enrolled in study (30 patients total) and the AF burden used for comparison has unclear clinical significance (<0.5% versus >0.5%). Consequently, we view this study as hypothesis-generating and look towards larger studies with longer follow-up periods to define the role of AF burden on cognitive function. Similarly, the role of catheter ablation as a means to improve long-term brain health and cognitive in patients with AF requires prospective long-term study and any preferential benefit over alternative therapies in a randomised controlled analysis.
Catheter Ablation of AF as a Risk Factor for Cognitive Impairment/Dementia
There are important potential risk factors that may increase risk of brain injury after ablation, many of which reflect ablation approach and subsequent management (Figure 2). Ablation is a destructive therapy that can decrease atrial compliance and function. Atrial functional impairment can persist years after even a successful ablation.48 Ablation is known to increase the risk of peri-procedural cranial embolic events from clots (e.g., denuded endothelium), char, air, etc. Although left atrial appendage isolation improves arrhythmia free survival, patients remain at an increased risk of stroke that may not be lowered completely with anticoagulation.49,50
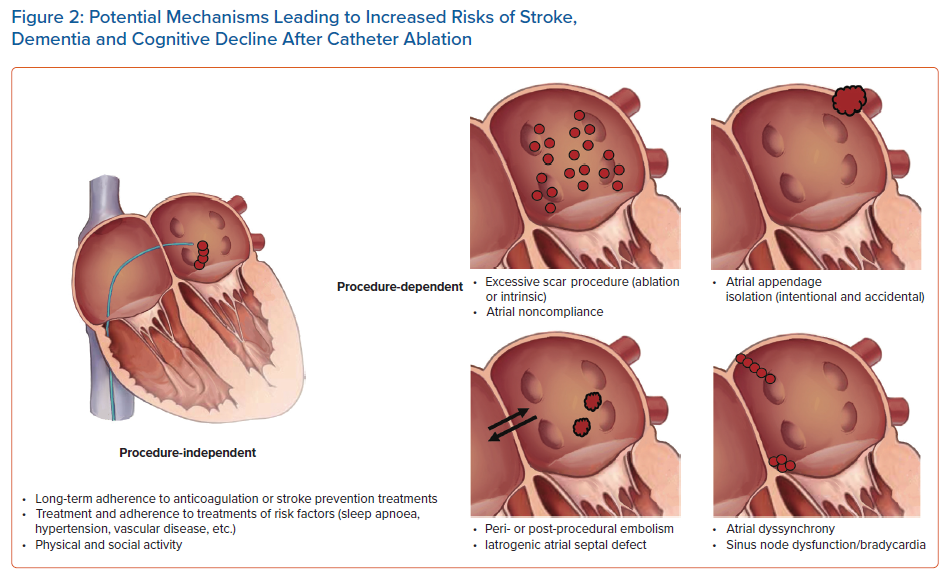
An alternative, left atrial appendage occlusion, has been advocated to be used in concert with appendage isolation procedures.51 A challenge with this approach is that these occlusion devices have never lowered embolic/ischaemic stroke rates compared to warfarin and require long-term study against direct oral anticoagulation.52 Additionally, there remains significant intermediate-term risk for device-related thrombus. Aggressive ablation approaches can cause right and left atrial dyssynchrony and as a consequence haemodynamic function and at times also result in severe bradycardia. Finally, rarely patients can have a persistent septal defect that raises risk of paradoxical embolus. Each of these potential risk factors for augmented risk of cognitive decline and stroke much be considered in all patients and also prompt the need to carefully consider ablation approaches and long-term patient management.
Conclusion
Outcomes after catheter ablation for AF are favourable and patients experience a better quality of life, arrhythmia-free survival and lower rates of hospitalisation compared to patients treated with antiarrhythmic drugs. When a patient is managed comprehensively for their AF and its associated comorbidities early in the disease state, then stroke rates also are reduced. In community settings, catheter ablation is consistently associated with lower rates of stroke compared to AF management without ablation in studies derived from large healthcare systems, national databases and across broad differences in healthcare utilisations and resources.
Recent observational data, including studies with specific cognitive testing, show that catheter ablation is also associated with a lower risk of cognitive decline and dementia that can be explained through a variety of pathways. Long-term, adequately powered randomised trials are required to define the role of catheter ablation in the management of AF to lower risk of cognitive decline, stroke and dementia.
Clinical Perspective
- AF has been consistently associated with multiple forms of dementia, including idiopathic dementia.
- Outcomes after catheter ablation for AF are favourable and patients experience a better quality of life, arrhythmia-free survival and lower rates of hospitalisation compared to patients treated with antiarrhythmic drugs.
- Catheter ablation is consistently associated with lower rates of stroke compared to AF management without ablation in large national and healthcare system databases.
- Multiple observational trials have shown that catheter ablation is also associated with a lower risk of cognitive decline, dementia and improved cognitive testing that can be explained through a variety of pathways.
- Long-term, adequately powered randomised trials are required to define the role of catheter ablation in the management of AF as a means to reduce risk of cognitive decline, stroke and dementia.










