It is now well established that there are two major pathways of conduction into the atrioventricular node, the so-called slow and fast pathways.1 It is also well established that ablation of one or other of the pathways can cure atrioventricular nodal re-entry. In this regard, the fast pathway is recognised as being closely related to the site of penetration of the conduction axis from the atrial to the ventricular myocardial components of the heart. For this reason, it is the slow pathway that is usually targeted during therapeutic ablation. However, the specific substrate that is ablated remains uncertain, although the site of ablation is now usually the septal vestibule of the tricuspid valve. Furthermore, atrioventricular nodal re-entry has now been shown to take various forms, which can be discriminated by electrophysiological investigations.2,3 Again, however, the specific pathways for the different circuits have yet to be established.
Much of the information regarding the existence of the dual nodal pathways has been derived on the basis of experiments using preparations from species such as the rabbit or dog. However, those making correlations between these results and the clinical observations have not always taken account of the significant differences in the arrangement of the atrioventricular node and its atrial connections between non-human and human species. This is somewhat surprising, because the differences between human and dog hearts were clearly described by Tawara when he clarified the overall arrangement of the atrioventricular conduction axis.4,5 Nonetheless, even though there are fundamental differences in morphological arrangements between species, the building blocks of the atrioventricular node are comparable.6
The differences in morphological arrangements can have a significant effect on the patterns of conduction into the atrioventricular node because they reflect the relationship between the node and the atrial vestibules and atrial septum. These differences, in turn, can now be related to the way in which the atrioventricular junctions are remodelled during normal cardiac development.
In this review, based on our recent investigations of human cardiac development and our access to a large number of serial histological sections of adult human hearts, we show how all these features can be combined to produce an understanding of the overall anatomy of the specialised atrioventricular junction.7 We are well aware that there is considerable information regarding the molecular and functional background of the specialised atrioventricular conduction axis. However, in this review we have confined ourselves to discussions of the basic template of the axis and the way in which it differs in humans compared with mammalian species used in electrophysiological experiments. It is our hope that the anatomical information provided here will set the scene for ongoing studies into the circuits involved in the different forms of atrioventricular nodal re-entry.8
Prescience of Sunao Tawara
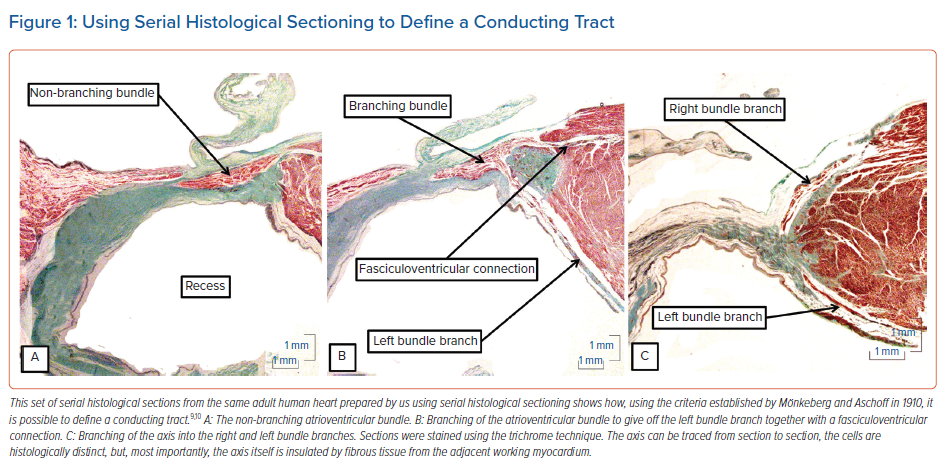
In 1906, Tawara provided a remarkably accurate account of the atrioventricular conduction axis, emphasising the different patterns to be found within several mammalian species.4 It is difficult to surpass this initial description, even with all the modern techniques at our disposal, such as immunocytochemistry and 3D reconstruction. Tawara had used the same approach that remains the mainstay for current anatomical investigations, namely the careful analysis of serially sectioned histological material. Similar analyses enabled Aschoff and Mönkeberg, the giants of cardiac pathology emerging at the beginning of the 20th century, to suggest criteria based on the initial studies of Tawara for the recognition of histologically specialised tracts within the walls of the cardiac chambers.9,10 It is these criteria that we have used as the basis of our own descriptions of the serially sectioned human datasets at our disposal.7 The criteria proposed by Aschoff and Mönkeberg were that:
- the structures in question should be traceable from section to section;
- the myocytes under consideration should be histologically distinct to the adjacent working myocardium; and
- of greatest importance for the recognition of conducting tracts, the myocytes, at least in the postnatal human heart, should be gathered together within an insulating sheath.
Applying these criteria to the serially sectioned histological dataset of adult human hearts meant that it was a relatively easy matter to recognise the non-branching atrioventricular bundle (Figure 1A), the origin of the left bundle branch from the axis (Figure 1B) and the continuation of the axis as it gives rise to the right bundle branch (Figure 1C). The significance of the criteria for the recognition of insulated tracts has recently been emphasised in the context of conduction through the working atrial myocardium.11
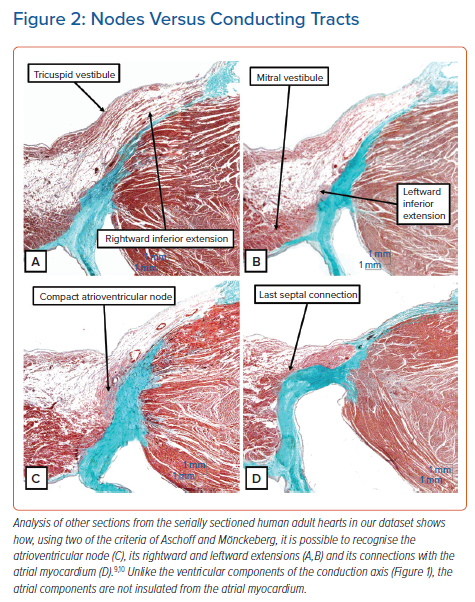
Using two of these criteria, namely the potential to follow the structures from section to section and the ability to distinguish specialised cardiomyocytes from the adjacent working myocardium, it is possible to recognise the components of the atrioventricular node (Figure 2). It is also possible to recognise cardiomyocytes that are intermediate in their staining characteristics between the working and specialised cardiomyocytes. These are the so-called ‘transitional’ cardiomyocytes. However, interrogation of our latest datasets revealed that these zones of transitional cardiomyocytes were less extensive in adult hearts than we had anticipated based on our earlier experiences.7
Unlike the components of the conduction axis as seen once it has penetrated the insulating tissues of the atrioventricular junctions, the myocytes of the atrioventricular node are not insulated from the adjacent working atrial myocardium. Insulation, of course, would defeat the very purpose of the atrioventricular node, which is to delay the transmission of the cardiac impulse to the ventricular myocardium while atrial contraction serves to fill the ventricles with blood prior to ventricular contraction. Nonetheless, it is an easy matter to recognise the compact atrioventricular node in histological sections (Figure 2C) using the criteria proposed by Aschoff and Mönckeberg.9,10 It is also possible to trace its extensions (Figures 2A and 2B) and, in particular, the arrangement of its connections with the atrial septal myocardium can be appreciated (Figure 2D).7 As emphasised above, in our initial experiences we had considered it possible to recognise discrete transitional areas between the working atrial myocardium and the specialised cardiomyocytes of the node and its extensions. We are now less convinced of the extent of such transitional cardiomyocytes.
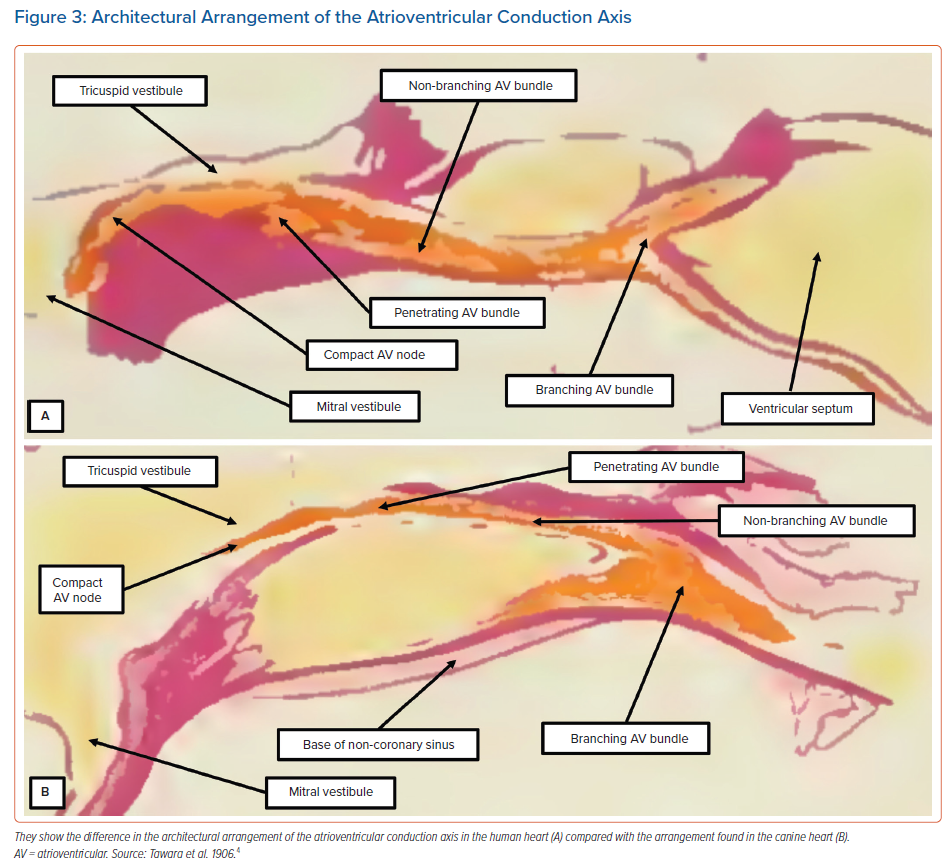
On the basis of his own studies using serial histological sectioning, Tawara had illustrated the marked differences in terms of the short extent of the non-branching bundle in the human heart compared with the much longer non-branching component found in the canine heart (Figure 3). There are also differences in this arrangement between the human heart and rabbit heart. In the rabbit, the most complex component of the conduction axis, in terms of its cellular architecture, is insulated from the adjacent atrial myocardium.12
We initially described this part of the axis as representing the ‘closed’ atrioventricular node.13 However, using the criterion proposed by Tawara,4 this part of the axis is properly defined as the non-branching atrioventricular bundle. Indeed, the rabbit heart does not possess a component of the atrioventricular conduction axis that can be compared in terms of its architecture with the compact atrioventricular node as observed in the human heart. Furthermore, in the canine heart, the atrial component of the conduction axis is much smaller than the structures typically found in human hearts. Therefore, it is surprising that subsequent investigators who used either dog or rabbit hearts to explore the electrophysiology of atrioventricular nodal function neglected to take note of these obvious differences, which are readily appreciated on the basis of the illustrations provided by Tawara in the first decade of the 20th century (Figure 3).
Developmental Considerations
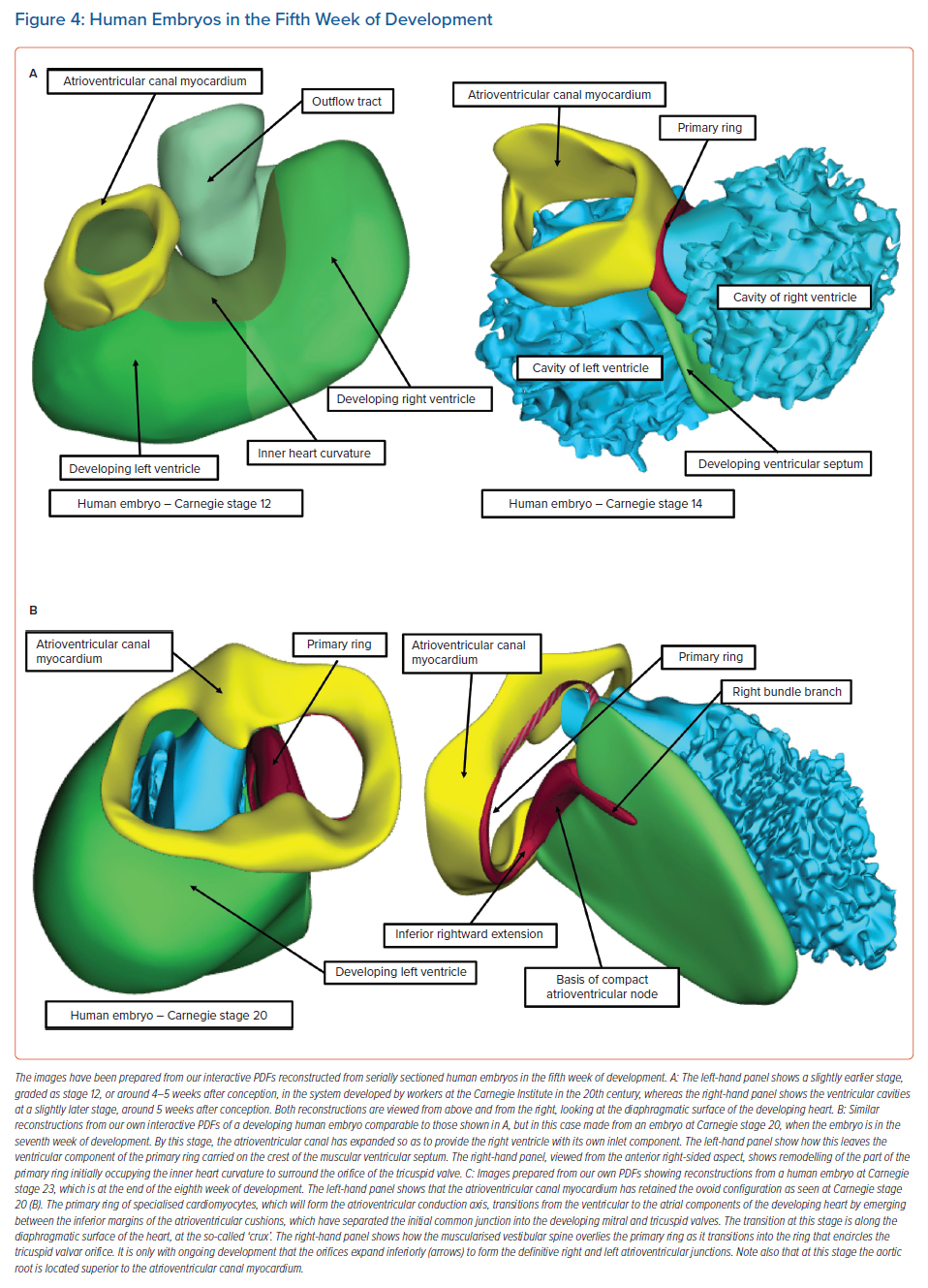
Knowledge of the development of the atrioventricular conduction axis now permits important inferences to be made regarding the potential substrates for atrioventricular nodal re-entry tachycardias.8 In the early stages of cardiac development, the developing atrial chambers are joined through the myocardium of the atrioventricular canal exclusively with the cavity of the developing left ventricle. All the blood entering the left ventricle must pass through the primary interventricular communication to reach the developing right ventricle, which supports the entirety of the circumference of the developing outflow tract (Figure 4A, left).
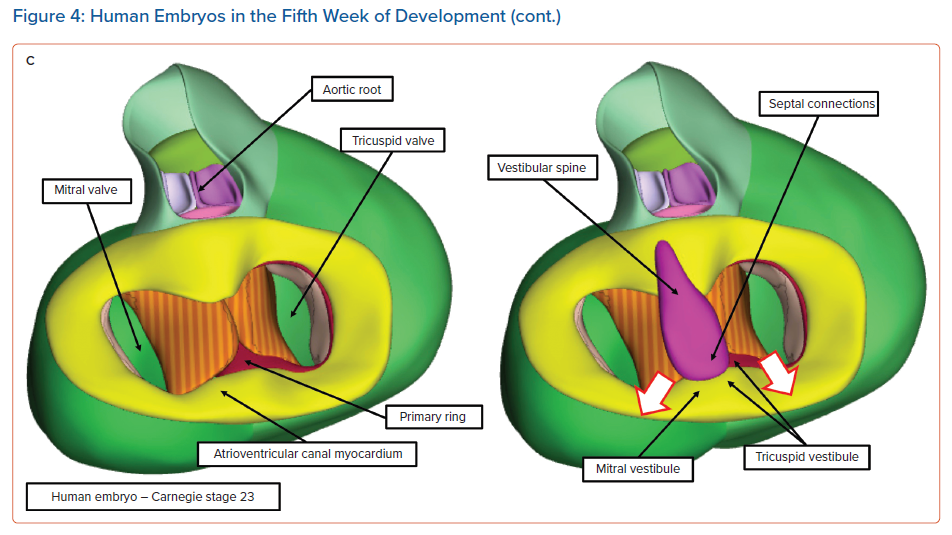
At this early stage, when the atrioventricular canal is supported by the developing left ventricle, it is possible to record an adult-type electrocardiogram, with evidence already present of atrioventricular delay.6 It is also clear that, even at this early stage, prior to the completion of septation, there is already a pathway of preferential atrioventricular conduction. The inference can be made that it is the atrioventricular canal myocardium that is producing the delay. At a slightly later stage of development, as shown in the right-hand image in Figure 4A, it is also possible to recognise a ring of cardiomyocytes encircling the interventricular communication. The cardiomyocytes react to an antibody raised against an extract of the nodose ganglion of the chick.14 These cardiomyocytes are carried on the crest of the developing ventricular septum and occupy the inner heart curvature, which, at this early stage of development, separates the atrioventricular canal from the developing outflow tract (Figure 4A). These cardiomyocytes are known as the primary ring and they form the basis of the atrioventricular conduction axis. They also occupy the right side of the developing atrioventricular canal. With ongoing development, the atrioventricular canal itself expands rightward, providing the developing right ventricle with its own inlet component. Concomitant with this rightward expansion, the part of the primary ring initially occupying the inner heart curvature is transformed so as to encircle the developing orifice of the tricuspid valve (Figure 5).
It is during the end of week 7 of human development and at the beginning of week 8 that the persisting channel between the aortic root and the developing right ventricle is closed by tubercles derived from the atrioventricular endocardial cushions; this process serves to complete ventricular septation.15 By this stage of development, another significant event has been the growth of a protrusion into the heart from the pharyngeal mesenchyme that muscularises, along with the mesenchymal cap carried on the leading edge of the primary atrial septum, to form the anteroinferior buttress of the atrial septum. This structure was described by Wilhelm His Senior in the latter half of the 19th century as the ‘spina vestibuli’ and was the largely forgotten prior to the study of Kim et al.16,17 It then received further attention from Snarr et al., who described it as the ‘dorsal mesenchymal protrusion’.18 Our preference is to continue to recognise this entity as the vestibular spine.19 The importance of this structure is to help bind together the middle of the developing atrioventricular junctions. As it grows into the atrial cavities, it fuses with the atrial surface of the atrioventricular endocardial cushions and the mesenchymal cap to close the primary atrial foramen. The cushions themselves have fused by this time to separate the initially common atrioventricular canal into the developing tricuspid and mitral valve orifices. Even after the canal has been separated into the right and left atrioventricular junctions, and after the vestibular spine has fused with the atrial surface of the endocardial cushions, the canal itself retains its initial ovoid configuration (Figures 4C and 5).
At the stage of development shown in Figure 4C, the primordial of the developing atrioventricular node are all in place, but are not yet in their final positions. An additional significant change is also taking place: with ongoing development, the atrioventricular canal myocardium is sequestered on the atrial aspect of the developing plane of insulation between the atrial and ventricular myocardial masses to form the vestibules of both the developing tricuspid and mitral valves.20 Subsequent to the formation of the insulating plane, the only persisting myocardial connection between the two masses is the atrioventricular conduction axis, which itself developed from the primary ring. As shown in Figure 4C, this axis transitions from the atrial to the ventricular components by passing between the inferior margins of the atrioventricular endocardial cushions. However, at the conclusion of the embryonic period of development (8 weeks in the human heart), there has been no inferior expansion of the right and left atrioventricular junctions. At this stage, the point of penetration of the axis, known as the cardiac crux, is found along the diaphragmatic surface of the developing heart. This is the point at which the primary ring, as it emerges from the ventricular mass, is overlain by the muscularising tissues of the vestibular spine and mesenchymal cap (Figure 4C, right). Therefore, at this early stage the transition from the ventricular to the atrial components (at the crux) marks the site of the formation of the atrioventricular node.
The nodal primordium then has three potential connections: with the vestibule of the tricuspid valve, with the mitral vestibule and with the atrial septal myocardium. The connection with the developing vestibule of the tricuspid valve involves not only the atrioventricular canal myocardium, which was itself sufficient initially to produce atrioventricular delay, but also the persisting parietal part of the primary ring, which is the same as the compact node itself. The second connection, with the mitral vestibule, is composed exclusively of the atrioventricular canal myocardium, because the primary ring encircles only the developing orifice of the tricuspid valve. Finally, the connection with atrial septal myocardium, formed by muscularisation of the vestibular spine and the mesenchymal cap and shown in Figure 4C, does not represent the definitive arrangement because, at the end of the embryonic period of development, there has been no expansion inferiorly of the right and left atrioventricular junctions, nor any commitment of the aortic root to the left ventricle. It is these ongoing changes taking place during the early foetal period of development that produce the inferior pyramidal space and the inferoseptal recess of the left ventricle.
Definitive Anatomical Arrangements
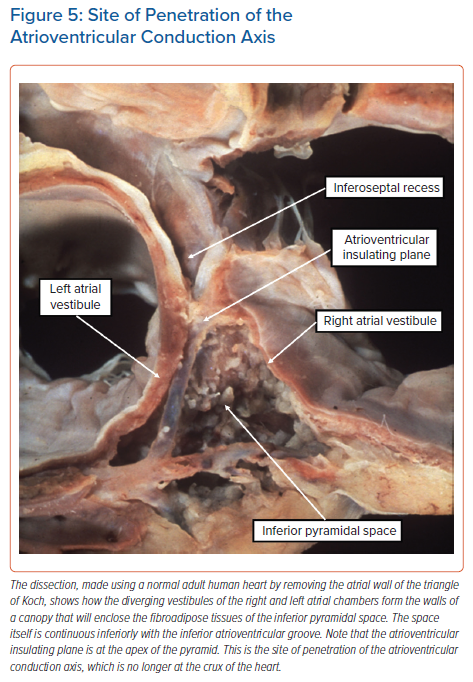
It is not coincidental that the arrangement of the developing conduction axis as seen at Carnegie Stage 23 (Figure 4C) is reminiscent of the arrangement found in atrioventricular septal defect with common atrioventricular junction.21 In the ‘ostium primum’ variant of these congenital lesions, the common junction is divided into separate right and left valvar orifices, but there has been no formation of the anteroinferior buttress of the atrial septum, with all these processes being the consequence of failure of the growth of the vestibular spine. The arrangement of the conduction axis in this setting is as shown in the left-hand panel of Figure 4C. It is the ongoing expansion, subsequent to normal development, of the mitral and tricuspid valvar orifices, along with the transfer of the aortic root to the left ventricle such that it interposes between the leaflets of the mitral valve and the septum, that establishes the normal arrangement of the atrial components of the atrioventricular conduction axis. The inferior expansion of the vestibules of the right and left atrioventricular junctions produces the inferior pyramidal space (Figure 5).
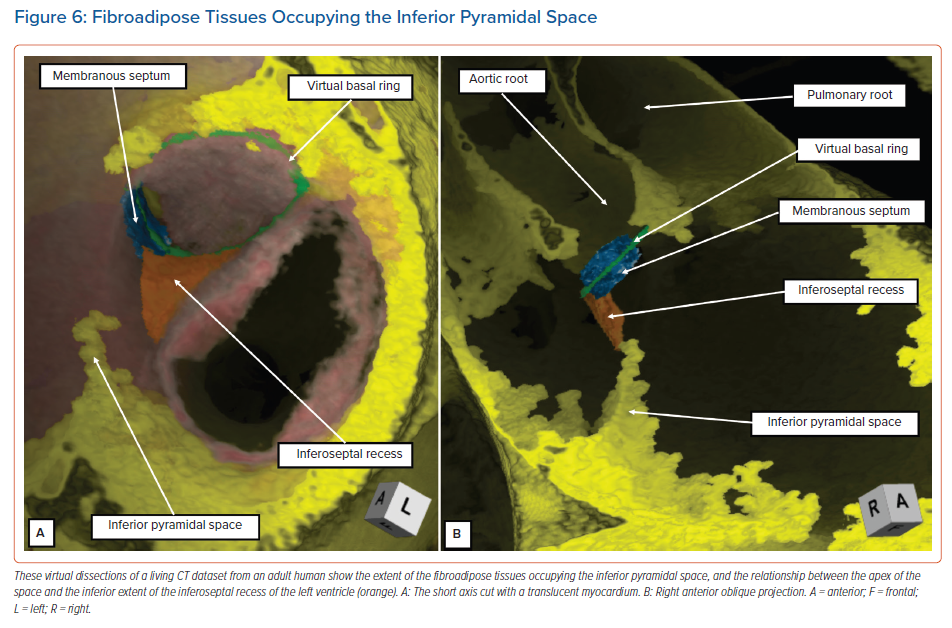
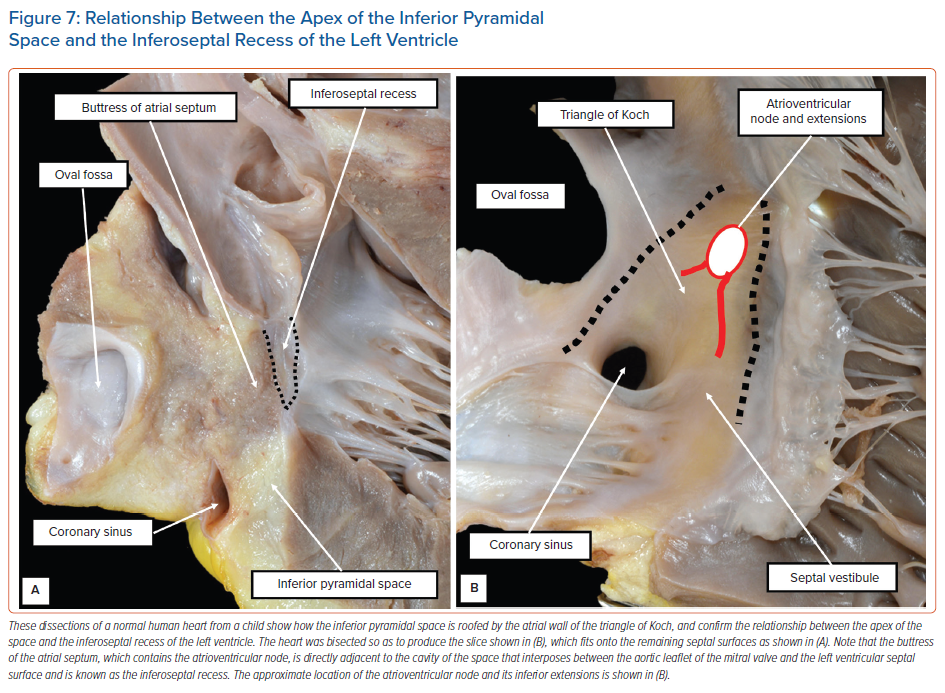
The diverging vestibules of the right and left atrial chambers, themselves formed from the initial atrioventricular canal myocardium, come together superiorly at the apex of the space. The atrial margin of the space is formed by the anteroinferior buttress of the atrial septum. At the apex of the pyramid, this contains the compact atrioventricular node itself. The space is continuous inferiorly with the inferior atrioventricular groove. The coronary sinus occupies the leftward part of this groove, with its mouth within the vestibular surface of the right atrium. These relationships are now readily demonstrated by virtual dissections of living CT datasets and show the importance of the relationship between the apex of the inferior pyramidal space and the inferoseptal recess of the left ventricle (Figure 6).
It is the extent of the relationship between the inferoseptal recess of the left ventricle and the apex of the inferior pyramidal space that determines the precise location of the atrioventricular conduction axis. As we have shown, the axis penetrates so as to provide the sole transition from the atrial to the ventricular myocardial masses at the apex of the pyramidal space. This is the point at which the initial primary ring of specialised cardiomyocytes extended from beneath the atrioventricular cushions to continue within the atrioventricular canal myocardium of the tricuspid vestibule as the ‘atrioventricular ring tissue’, this particular ‘ring tissue’ being derived from the primary ring.22 It is only the tricuspid vestibule that contains the remnants of the ring tissues derived from the primary ring. These tissues form the rightward inferior extension of the atrioventricular node. However, the compact atrioventricular node is contained within the anteroinferior buttress of the atrial septum. The vestibular spine is an interatrial and atrioventricular septal complex, rather than a right atrial structure. The septum itself, derived by muscularisation of the vestibular spine and mesenchymal cap, is supported by the roof of the inferoseptal recess of the left ventricle, formed by an area of fibrous continuity between the leaflets of the mitral and tricuspid valves (Figure 7).
The atrial component of the conduction axis penetrates the fibrous tissue, forming the roof and rightward wall of the inferoseptal recess to become the bundle of His. It then continues within the rightward wall of the recess as the non-branching atrioventricular bundle. As emphasised, the node itself, prior to penetration of the axis, is part of an atrioventricular septal complex. However, its extensions belong separately to the vestibules of the right and left atrial chambers. Nonetheless, its septal connections are self-evidently contained within the anteroinferior buttress of the atrial septum. These three building blocks of the nodal connections (i.e. the two inferior extensions and the septal connections) are present in all hearts. Their specific arrangement, and extent, varies markedly. This was revealed by our analysis of the histological datasets prepared from normal adult human hearts.7 We are able to illustrate the significance of the variations using selected cases from our series.7
Variations in Nodal Building Blocks
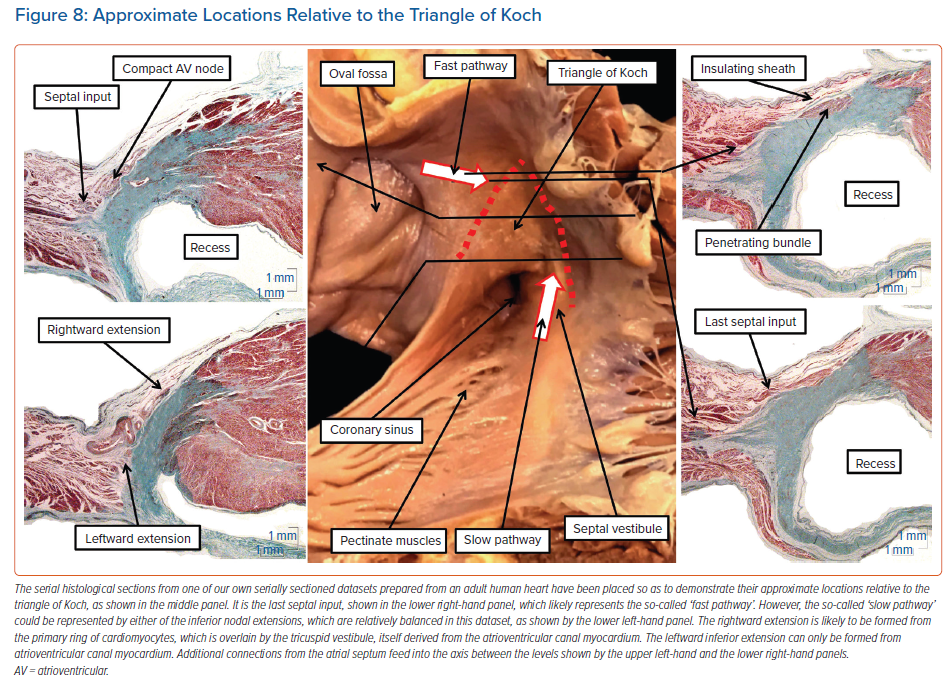
The atrioventricular node itself varies in its position within the triangle of Koch. We had initially believed the node to be found at the apex of the triangle. This is still a good general guide to its position. However, our combined histological and electrophysiological assessment showed much greater variability in this location than we had initially anticipated.23 We also found variation in terms of whether the compact node itself was supported by a plate of fibrous tissue formed at the apex of the inferior pyramidal space or whether it was incorporated on the right wall of the inferoseptal recess as part of an atrioventricular fibromyocardial septal complex. Regardless of these considerations, the compact node itself is unequivocally a septal structure. As emphasised, it then potentially has right atrial and left atrial extensions, along with additional direct septal connections (Figure 8).
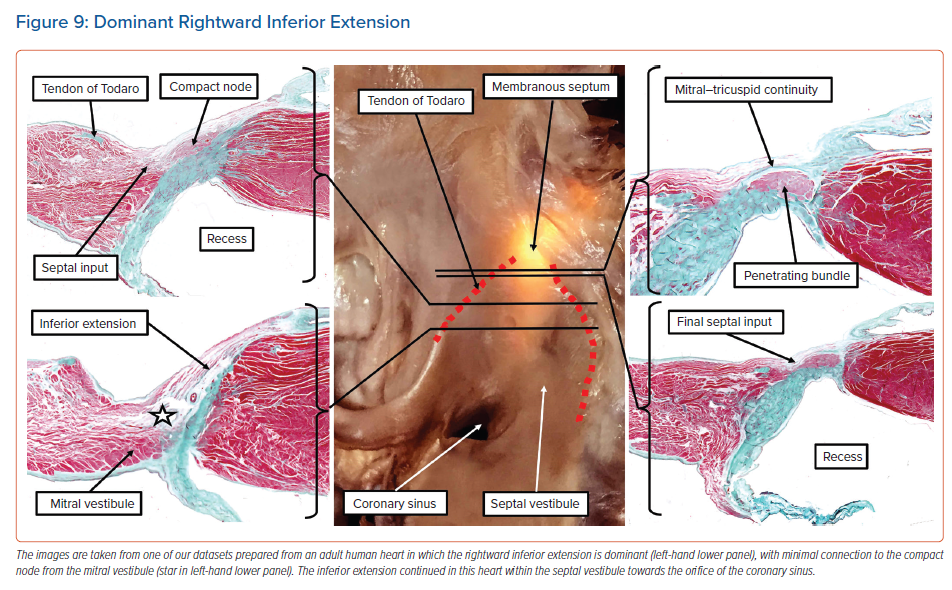
The dataset as shown in Figure 8, prepared from an adult human heart, illustrates the three basic ‘building blocks’ in regard to the atrial cardiomyocytes that make contact with the specialised cardiomyocytes forming the compact atrioventricular node. Of the 20 human datasets we analysed in detail to assess these connections, which came from normal adult hearts, only two showed the balanced arrangement as illustrated in Figure 8.7 Most of the datasets showed dominance of the right inferior extension.7 As we have emphasised, this extension is almost certainly the remnant of the specialised cardiomyocytes derived from the primary ring and incorporated into the tricuspid vestibule. As can be seen, the extension itself is overlaid by the working myocardium of the tricuspid vestibule, which itself is a remnant of the embryonic atrioventricular canal myocardium. Even in those datasets in which the rightward inferior extension is the dominant pathway feeding the inferior part of the compact node, there is still further variation of the extension itself within the vestibule. In some of the hearts, the extension cannot be followed for any significant distance as an entity distinct from the vestibular myocardium. In other hearts, the histologically discrete cardiomyocytes, fulfilling one of the criteria established by Aschoff and Mönckeberg for the recognition of ‘nodal’ tissue,9,10 extend within the septal vestibule towards the orifice of the coronary sinus, but are again overlaid by the vestibular myocardium (Figure 9).
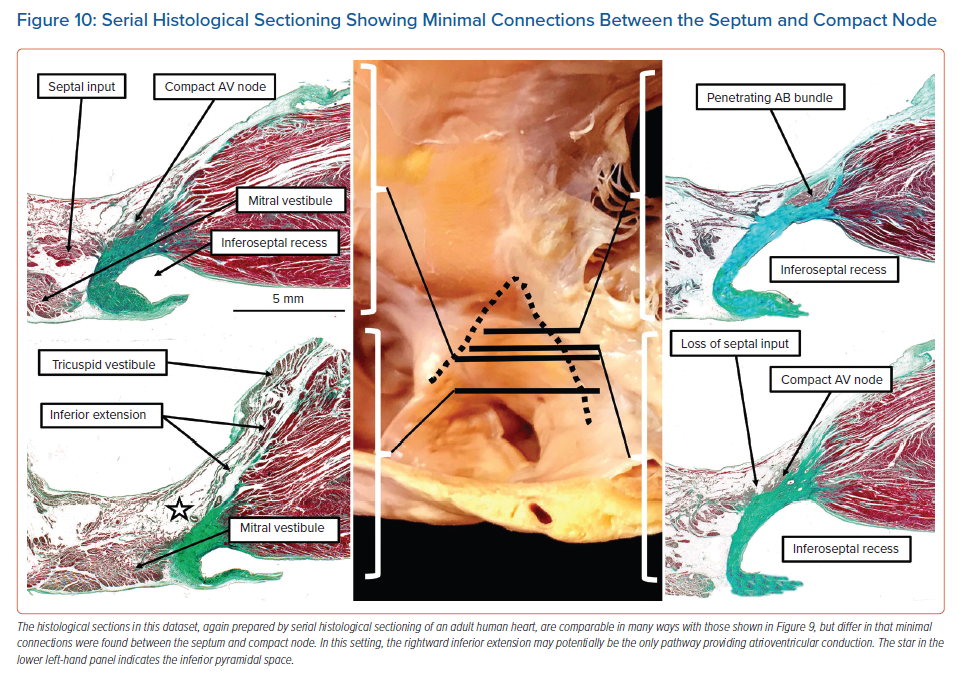
In still other datasets, we found fibroadipose tissue within the inferior pyramidal space disrupting the connections between the septal cardiomyocytes and the compact node. This arrangement would potentially leave the rightward inferior extension itself as the solitary pathway into the compact atrioventricular node (Figure 10).
Composition of the ‘Ring Tissues’
In this review, we have emphasised the presence of three ‘inputs’ to the compact atrioventricular node, namely the rightward and leftward inferior extensions, and the direct connections from the atrial septum. We have also highlighted the need to distinguish between the findings in experimental animals and the arrangement in the human heart. This latter point is relevant when we assess the relationships between the connections themselves and the so-called ‘ring tissues’. We first described the ‘ring tissues’ in the human heart as remnants of the primary ring, pointing out that it was these remnants that almost certainly persist as the structures incorrectly judged by Kent as producing accessory atrioventricular myocardial pathways in the normal heart.24
We subsequently showed that, on occasion, the remnants could indeed persist as part of a re-entry circuit, forming the atrial component of the pathways nowadays described as atriofascicular tracts.25 These cardiomyocytes are part of the tissues we have described as the ‘primary ring’. As we have shown, they surround the developing orifice of the tricuspid valve, forming the basis of the compact node, but significantly also its rightward inferior extension. Therefore, the cardiomyocytes of the primary ring form one of the inputs to the atrioventricular node. These cardiomyocytes are overlaid by the cardiomyocytes of the tricuspid valvar vestibule, which themselves are derived from the atrioventricular canal myocardium.17 This is another ‘specialised’ area of myocardium, because it is known that it is the atrioventricular canal that provides the necessary delay in conduction of the cardiac impulse in many animal species, as well as early during cardiac development in mammals.6
Furthermore, it is the atrioventricular canal myocardium that was identified as an additional ‘ring’ of specialised cardiac tissue in the investigation of Yanni et al.22 This second ring, identified using immunocytochemical markers in hearts from several non-human species, provides not only the tricuspid vestibular myocardium, but also the myocardium of the mitral valvar vestibule, and it is the mitral vestibule that produces the second inferior extension from the atrioventricular node. Therefore, both inferior nodal extensions are histologically specialised, but the rightward extension, derived from the primary ring, is additionally associated with the tricuspid valvar vestibule, derived from the ring derived from the atrioventricular canal myocardium. Either of these extensions may be presumed to be capable of slow conduction. Therefore, it is appropriate that one or other, or both, is believed to be a potential substrate for the slow pathway.1,8 The leftward extension may be long enough to reach the inferoposterior aspect of the mitral vestibule.26 In contrast, the septal connections of the compact node are derived by muscularisation of the vestibular spine and the mesenchymal cap. They are presumably working atrial cardiomyocytes. It is the final of these connections that is now suggested to function as the fast pathway.7 Hence, the inferences to be made from cardiac development, and the structure of the atrioventricular node, add support for developing theories regarding the potential circuits for the different types of atrioventricular nodal re-entry.
The compact atrioventricular node itself is formed at the inferior end of the transition of the primary ring from the ventricular to the atrial myocardial masses. During initial cardiac development there is a second superior transition, which is broken during ongoing development with the formation of fibrous continuity between the leaflets of the aortic and mitral valves, this process attenuating the myocardium of the inner heart curvature. The atrial remnant of this second transition forms the retroaortic node.22,27 This nodal remnant, at least in human hearts, is separated by an area of working myocardium from the compact atrioventricular node. Its precise function, if any, remains to be clarified.27,28
Conclusion
On the basis of our findings during cardiac development, we can predict that all hearts have the basis for the compact atrioventricular node to make contact with atrial cardiomyocytes via its right and left inferior atrial extensions, or via the cardiomyocytes of the atrial septum. It is these connections that must provide the anatomical substrates for the ‘slow’ and ‘fast’ pathways into the node. As yet, electrophysiologists have been unable to characterise the specific composition of these pathways.8 Our analysis of datasets prepared from normal adult human hearts showed marked variation in the extent of these potential substrates. Furthermore, their architecture was subtly different from the arrangements found in mammalian species used in experiments designed to reveal the physiology of these presumed pathways. The increasing ability to assess the composition of the inferior pyramidal space, combined with knowledge of potential variations, will hopefully soon lead to clarification of the different pathways underscoring nodal re-entry tachycardia.
Clinical Perspective
- In all hearts, the compact atrioventricular node can make contact with atrial cardiomyocytes via its right and left inferior atrial extensions, or via the cardiomyocytes of the atrial septum.
- It is these connections that provide the anatomical substrates for the so-called ‘slow’ and ‘fast’ pathways into the node.
- The increasing ability to assess the composition of the inferior pyramidal space, combined with increasing knowledge of potential anatomical variations, increases the likelihood that the arrangement of the different pathways underscoring nodal re-entry tachycardia will soon be clarified.
- The role of the retroaortic node as a potential tachycardia substrate remains to be established.