The Electrocardiogram
In the entire realm of his work, Dr Josephson’s greatest love was the simple electrocardiogram (ECG). His discernment, based on the ECG, into the patient’s history and disease processes was legendary. Among the more important and insightful contributions he made to our understanding of heart disease and arrhythmias from the ECG are: observations of alternating preexcitation patterns in patients with Wolff-Parkinson-White (WPW) pattern who had more than one accessory atrioventricular (AV) accessory pathway (AP),1 and a patient with 1:2 conduction AV using an AV pathway;2 the nature of electrocardiographic ‘left atrial enlargement’;3 multiple ECG manifestations of ventricular tachycardia (VT) in the same patient;4,5 use of the 12-lead ECG to localise exit sites of post-infarction VT6,7 and premature ventricular complexes;8 studies on electrical alternans in wide complex tachycardias;9 a highly useful method of distinguishing VT from supraventricular tachycardia (SVT) with aberration in left bundle branch block (LBBB) wide-QRS tachycardias;10 variations in the ECG expression of atrial flutter;11 differentiation of insertion sites of atriofascicular pathways;12 classification of which portion of which coronary artery was affected in myocardial infarction;13,14 features of the ECG in what appears to be right ventricular outflow tract ectopy and VT that correlate with difficult catheter ablation;15 distinction of the causes of T-wave inversion (post-pacing cardiac memory versus ischaemia),16 and whether LBBB was new or old (with implications for patients with chest pain and LBBB on ECG).17
Throughout his career, Dr Josephson gave courses on ECG interpretation with his close friend Prof Hein Wellens; most EP fellows of the last 25 years have attended these legendary courses and come away with a new appreciation of this old tool and how much information can be extracted by masters such as Josephson and Wellens. In the later years of his career, he was invited along with Prof Wellens to provide a regular contribution to the prestigious journal Heart Rhythm – ‘Josephson and Wellens’ ECG Lessons: A Monthly Visit to the 12-Lead ECG’. Here, they retrieved some of the choice jewels from their treasures of ECGs obtained over the prior half-century, sharing their insights into how the ECG could yield important and often surprising information about the patient and their proper diagnosis and management. These pearls bear careful reading by the serious student of electrophysiology. Josephson was often heard to state something like the following: “The ECG never lies, but it doesn’t always tell you all it knows.”
A couple of anecdotes are worth sharing here. In preparing our 1988 paper on correlation of the 12-lead ECG with ‘site of origin’ of VT, some of the ECGs had been recorded as a long continuous strip from which I had to cut several complexes of individual leads and affix them to paper in a standard 12-lead ECG format. In one case, I had inadvertently mounted a lead V5 upside down; as I was reviewing a stack of these ECGs with Dr Josephson, he immediately noted the error. I was of course very embarrassed and apologetic but he was not as bothered by it as I was, knowing what it should look like. He could also name from which patient most of the ECGs came. In another instance, during a regular Monday ECG conference in a darkened room, a fellow was squirming uncomfortably at the front of the room trying to analyse an ECG projected on the screen that showed an atrial bradycardia, incomplete right bundle branch block and mild right ventricular hypertrophy. At that moment, Dr Josephson burst into the room looking for one of the other fellows; he glanced at the ECG on the screen and blurted out the diagnosis (sinus venosus atrial septal defect) and the name of the patient (one of his). Such was his command of the ECG. Although most cardiologists interpret the ECG well, and some even interpret it expertly, Dr Josephson interpreted and understood the ECG deeply, as do but a few others.
Supraventricular Arrhythmias
Dr Josephson started his investigative career with supraventricular arrhythmias such as SVT and atrial fibrillation (AF) and flutter. In a general overview on paroxysmal SVT18 – on which he was the sole author – he showed even this early in his career (1978) how extensive his understanding of this family of arrhythmias was (as well as how much was still unknown). Among his favourite arrhythmias was AV nodal reentrant tachycardia (AVNRT), on which he published extensively throughout his career. The first of these studies investigated whether the atrium was a necessary component in AV nodal reentry.19 Following this were investigations into upper and lower common pathways between the tachycardia circuit and atrium (above) and His bundle (below);20,21 using the ΔHA interval (HA during SVT versus HA during ventricular pacing at the SVT rate) to distinguish AV nodal reentry from orthodromic SVT using a septal AP;22 variations in retrograde conduction pathways in AVNRT, in which he showed that retrograde conduction did not necessarily have the same atrial activation pattern during SVT and retrograde conduction with ventricular pacing;23,24 the origin of accelerated junctional rhythm during slow pathway ablation for AVNRT;25 that standard slow pathway ablation of AVNRT suffices in patients with earliest retrograde activation in the coronary sinus;26 the unusual rhythm disturbance of 1:2 AV conduction during sinus rhythm coexisting with typical AV nodal reentry (which many thought impossible);27 and his final major work on AVNRT, in which he matter-of-factly corrected some of his own prior work in the light of newer evidence.28 Dr Josephson also added to knowledge and practice in a paper on selection of sites for ablation of APs in WPW, in particular using the unipolar electrogram;29 the previously-noted atriofascicular AP insertion study;12 and several papers investigating AF. These included evaluating the success of AF ablation by pulmonary vein (PV) isolation when entrance block into, and exit block out of, the PV were obtained,30,31 as well as correlating acute PV reconnection after ablation with poorer outcomes;32 a case in which AF was contained within a PV after isolation;33 and pointing out the frequency of aortic wall injury during AF ablation procedures.34 From the start of his career to its end, despite his plethora of other investigative interests, his attention was never very far from SVTs.
Ventricular Tachycardia
The area of clinical investigation for which Dr Josephson is most renowned is VT. In the 1970s, post-infarction VT was a major problem without a good solution. Antiarrhythmic drugs were palliative at best, and neither catheter ablation nor implantable defibrillators had been developed. Dr Josephson believed that effective treatment might hinge on a better understanding of the nature of the arrhythmia – which at that time was poor. He and his colleagues began a series of investigations that not only changed many aspects of the care of patients with VT, but eventually those with many other types of arrhythmias (to which the principles learned in VT investigation could be applied). The first studies he published in this area were an astounding series of four landmark papers, appearing in Circulation over a span of 13 months.4,35–37 This was a planned series (the first is entitled ‘Recurrent Sustained Ventricular Tachycardia. 1. Mechanisms’) setting forth the state of the art at that time, and showing that:
- VT in this setting was due to reentry;
- Reentry occurred on the endocardium;
- Reentry did not involve the proximal His-Purkinje system; and
- Reentry occurred in a relatively small area.
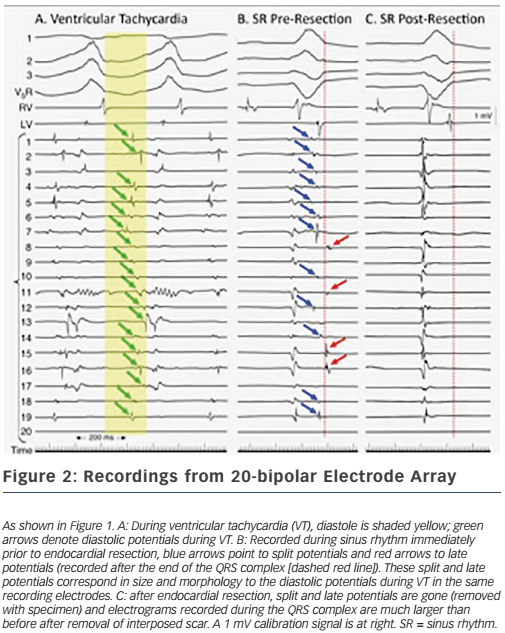
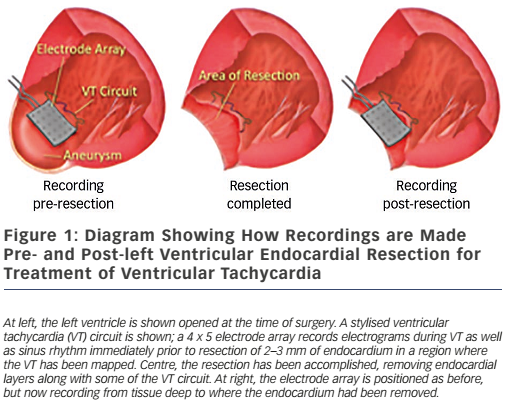
These findings led directly to surgical treatment of VT by excising endocardial tissue proven to be essential for ongoing arrhythmia; finally, a cure for VT was possible.38,39 A series of papers followed this providing further insights into the behaviour of VT5,40,41 – where circuits were, patterns of activation during VT, what accounted for multiple QRS morphologies of VT, observations about ‘dead-end’ conduction pathways with 2:1 conduction during VT – and led to innovations in technique, resulting in improved outcomes.42 This, in turn, paved the way for subsequent work in catheter ablation of VT, in which Dr Josephson was an active investigator.43 Additional investigations involved the aforementioned use of the ECG during VT to localise exit sites from circuits,6,7 pace-mapping to corroborate activation mapping;44,45 and one of his favourite studies, the effect of endocardial tissue resection on electrograms at sites of reentry.46 In this study, a multipolar electrode array was positioned on a portion of endocardium to which VT had been mapped during the procedure; recordings were made during sinus rhythm (and usually also VT); the endocardium was then removed by undermining with scissors; and finally, the array was placed back in the same position where recordings were repeated during sinus rhythm (see Figure 1). This showed that endocardial late potentials (recorded after the end of the QRS complex) that correlated with mid-diastolic potentials during VT were located on the endocardial surface (present prior to resection, absent after), and that the larger signals seen during the QRS complex were recorded from deeper layers, with their amplitudes decreased by endocardial scar (see Figure 2). He also participated in studies of VT in the absence of structural heart disease47 as well as in the setting of congenital heart disease (repaired tetralogy of Fallot, Ebstein anomaly).48,49 One of his last battles against VT was in the form of prophylactic catheter ablation of VT to try to prevent shocks from implanted defibrillators;50 he always believed prevention was a better strategy than rescue (analogy: preventing injury by preventing a motor vehicle accident, versus rescue by airbag), so this study was a natural fit for him.
Mechanisms and Tools of Investigation
Dr Josephson was known for his earnest search for a more thorough understanding of the mechanisms of arrhythmias, strongly believing that correct therapy depended on knowing the correct diagnosis. While this seems self-evident, some electrophysiologists still perform catheter ablation procedures based on a presumption of a diagnosis and mechanism (reentry versus automaticity, for instance), rather than taking the extra few seconds or minutes needed to prove the case. A long and ultimately unsuccessful procedure can result from a strategy of presumption, if incorrect. Dr Josephson penned two perspective papers entitled ‘Electrophysiology at a Crossroads’51,52 that set forth his reservations about how EP practices had evolved from mechanismand evidence-based care to what was expedient and economically driven; these are required reading in many training programmes.
Dr Josephson’s research into mechanisms of arrhythmias was spread throughout his career, starting with a publication on the effects of lidocaine in man.53 A few years later, the previously-mentioned work on mechanisms of VT appeared as the first of many regarding this arrhythmia. Following this were papers describing continuous electrical activity during VT;54 the mechanism of ventricular fibrillation;55 structure and ultrastructure of surgically-removed specimens from VT patients;56 and the meaning of endocardial late potentials.57 A series of monumental papers on resetting and entrainment of arrhythmias began in 198558 and continued for a decade thereafter, including resetting response patterns;59,60 resetting in the presence of fusion;61 comparing results of single-beat resetting versus entrainment;62 predictability of overdrive pacing to terminate VT;63 and investigation as to whether functional or fixed lines of conduction block are present in VT.64 The lessons learned from these studies provide substantial help in some cases of complex reentry, which are increasingly encountered after ablation of AF. Dr Josephson provided a lucid explanation of the mechanism of the rare but important disorder known as paroxysmal AV block.65 He also contributed to the development of the first electroanatomical mapping system.66
Dr Josephson participated in investigations into drug therapy of arrhythmias and the adverse effects of these drugs; cardiovascular genetics; animal models of arrhythmias; heart failure; valvular heart disease; device-based therapy of atrial and ventricular arrhythmias; clinical trials in arrhythmia therapy; and healthcare economics.
Conclusion
The panoply of contributions by Dr Josephson to our understanding of cardiac arrhythmias is far too broad to recount in a short paper. Even in failing health in his last two years here, he continued to develop new ideas about ways to investigate arrhythmias. His contributions to the field are large, both in number and impact; practically every procedure that a practicing electrophysiologist does on any given day has been influenced by Dr Josephson’s work. These accomplishments were of course important to him; far more important were his relationships with his family, colleagues and trainees. Ever the advocate and friend, he never saw himself as too important or busy to help someone understand a difficult point, or offer patient care or career advice when asked. A good clinical investigator does good work; an exceptional investigator inspires those with whom he works to launch out onto their own investigative paths. Dr Josephson was clearly among the exceptional. Thus it is that, through his students – who he taught how to think and ask questions – his work continues.
Lastly, although Dr Josephson made many new discoveries and devised new therapies in his investigative career, some of the conclusions he made from these studies turned out to be less than completely correct; he was big enough to own up to these and criticise his own prior work. A pioneer in any field, like an explorer in new territory, occasionally goes down a seemingly promising path that eventually leads nowhere. Far more often than not, however, Dr Josephson led us down a path of new discovery and we find, in looking back, that the ‘hits’ far outnumbered the ‘misses’. It is difficult to imagine where our knowledge about arrhythmias would be, and how we would be practicing EP today, were it not for the contributions of this one man. His legacy as a pioneer in our field is secure.