Until recently, the vitamin K antagonist (VKA, e.g. warfarin) class of drugs was the only oral anticoagulant in use. VKAs have important inter- and intra-patient variability, influenced by diet, alcohol and drugs; thus, regular anticoagulation monitoring is necessary. Indeed, VKAs offer their best efficacy and safety when the average time in therapeutic range (TTR) is >65–70 % in a particular individual.1,2
In the last decade, we have witnessed the emergence of the oral non-vitamin K oral anticoagulants (NOACs). NOACs have numerous advantages compared with the VKAs, particularly their lack of need for monitoring; as a result their use is increasing. Nonetheless, the NOACs face two major challenges: the need for reliable laboratory assays to assess their anticoagulation effect and the lack of approved antidotes to reverse their action.
This article provides an overview of monitoring the anticoagulant effect of NOACs and their potential specific antidotes in development.
Significance of the NOACs
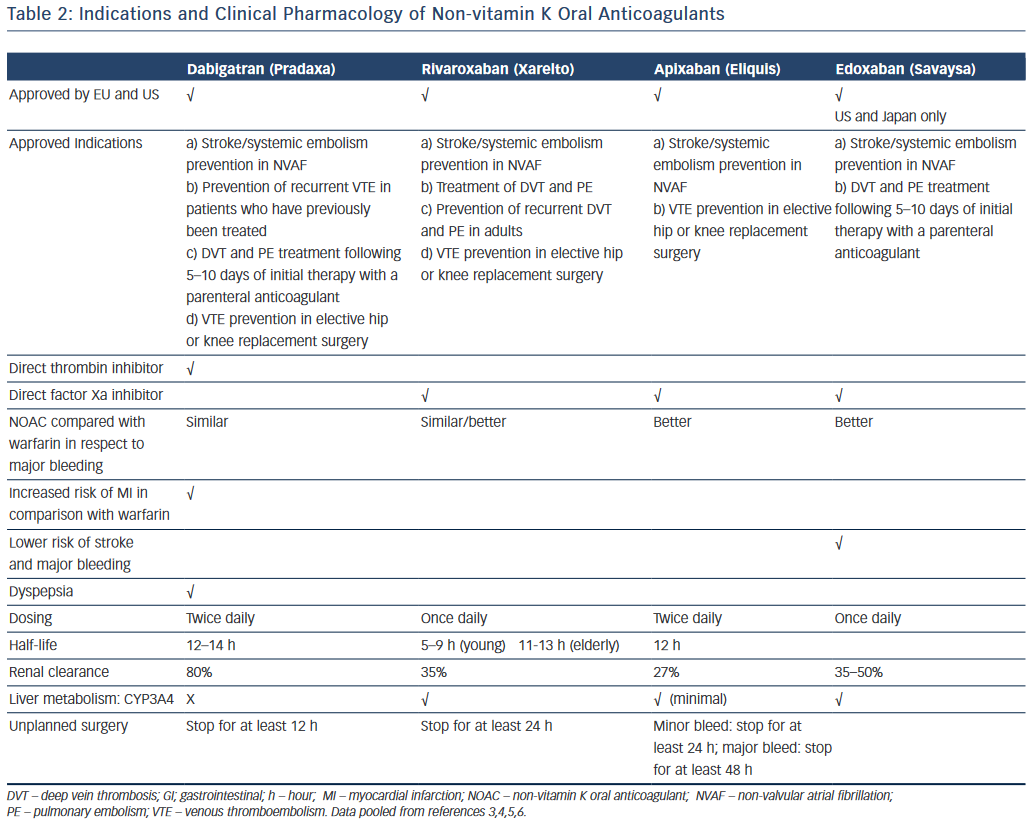
NOACs have been introduced as alternatives to warfarin for various thromboembolic indications; including prevention and treatment of venous thromboembolism, stroke prevention in atrial fibrillation and secondary prevention in high-risk patients presenting with an acute coronary syndrome.3–7 The main advantage of the NOACs is their lack of need for routine blood monitoring. Furthermore, they have relatively few food–drug interactions. Other advantages of NOACs include predictable efficacy, rapid onset of action and fixed dosing.8 The large randomised trials with NOACs have now been complemented by large real-world observational data showing the relative efficacy and safety compared with warfarin.9–13
There is no role for routine monitoring to assess efficacy of NOACs. However, the ability to monitor the anticoagulant effect of NOACs can be helpful in selected situations including severe bleeding and thrombotic complications, urgent/emergent invasive procedure or surgery, suspected drug failure, overdose, treatment compliance and in special situations such as in individuals who are elderly, have renal/ liver dysfunction or extremes of body weight.14
There are no validated quantitative assays or coagulation assays that are adequately sensitive to the NOACs. Current available laboratory tests are a practical selection as they are globally available and easily accessible. They measure the global status of the coagulation system in a patient, but not their precise plasma concentrations. Thus, these tests provide an indication of the anticoagulation effect rather than anticoagulation intensity.
Monitoring Assays for the NOACs and their Diagnostic Value
To calculate and predict the anticoagulant activity, six key components need to be borne in mind: 1) choice of coagulation tests differ by clinical objective, 2) results often take >2 hours, 3) some tests may not be available in all institutions, 4) timing of the last dose is important, 5) quantitative or qualitative assays can be used and 6) kidney insufficiency. Cuker et al have systematically reviewed up to 17 articles on dabigatran, rivaroxaban and apixaban, which have demonstrated variable effects on coagulation assays.15
Direct Thrombin Inhibitor: Dabigatran
Dabigatran prolongs most coagulation assays except prothrombin time (PT).16 The interpretation of these tests is highly dependent on the reagents used.
Activated Partial Thromboplastin Time (aPTT). Dabigatran prolongs the aPTT in a curvilinear relationship; dose response curve is linear up to a concentration of 200 to 300 ng/ml and then flattens out at higher drug levels.17 For this reason, aPTT is unsuitable for quantitative measurement.17–20 Commercial aPTT reagents differ widely in their sensitivity to dabigatran; thus, their calibration standards and interpretation should be communicated to clinicians. For qualitative measurement, especially during an emergency, the aPTT may help determine if dabigatran contributes to a haemorrhagic event. At trough (12–24 hours postdose), an aPTT level two times the upper limit of normal may indicate higher risk of bleeding.17
Thrombin Time (TT) is measured at 12–24 hour trough levels. The TT assay may serve as a sensitive method for determining the presence of dabigatran: a normal TT excludes a dabigatran-associated bleeding risk.17,18 However, TT is exquisitely sensitive to the presence of dabigatran and it may be too sensitive in the clinically relevant plasma concentration range.15 Depending on the reagent, TT frequently exceeds the maximum measurement time of the coagulometer.19,20
The Dilute Thrombin Time (dTT) displays a high degree of linearity with drug plasma concentration and thus is useful for quantitative measurement. A dTT overcomes the excessive sensitivity of the TT. A normal dTT indicates no clinically relevant anticoagulant effect of dabigatran.18 The dabigatran-calibrated Hemoclot® thrombin inhibitor assay (a dTT) is commercially available for use to estimate drug level, although it is still not widely available.21,22
The Ecarin-based Assays, ecarin clotting time (ECT) and ecarin chromogenic assay (ECA), show a high degree of linearity with drug plasma concentrations in the clinically relevant drug concentration range.15 They exhibit adequate sensitivity and precision. At trough, more than three times elevated ECT is associated with a higher risk of bleeding.23 However, ECT and ECA are not readily available in many clinical settings due to lack of standardisation, variability in sensitivity to dabigatran among different samples of ecarin, and limited availability.19,24
The Prothrombin Time (PT), or international normalised ratio, is unsuitable for measuring the effects of dabigatran due to its low sensitivity and substantial variability.25
Direct Factor Xa Inhibitors: Rivaroxaban and Apixaban
Specific Anti-factor Xa Chromogenic Assays, which are distinct from low molecular weight heparin (LMWH) testing, are the rational choice for measuring plasma concentrations of direct factor Xa inhibitors.They are calibrated individually for each drug (i.e. rivaroxaban, apixaban and likely edoxaban) and expressed in mass concentration (e.g. mg/l).26–28 In general, anti-factor Xa activity measurements demonstrate a strong linear relationship with rivaroxaban (up to a concentration of 500 ng/ml) and apixaban (at all concentrations).
Prothrombin Time. Rivaroxaban has been shown to prolong the PT in a concentration-dependent and linear fashion.29–31 However, the results are significantly dependent on the PT-specific reagents used in the assay (e.g. neoplastine). A normal PT does not exclude clinically significant rivaroxaban concentrations; however, a prolonged PT qualitatively indicates the drug’s presence.32
Apixaban has demonstrated less of an effect on the PT than rivaroxaban and it is not recommended to assess the pharmacodynamic effects of apixaban.18 Currently, there is still no validated coagulation assay available to measure the anticoagulant effect of apixaban.33
Expert Opinion: What Should We Use?15,18
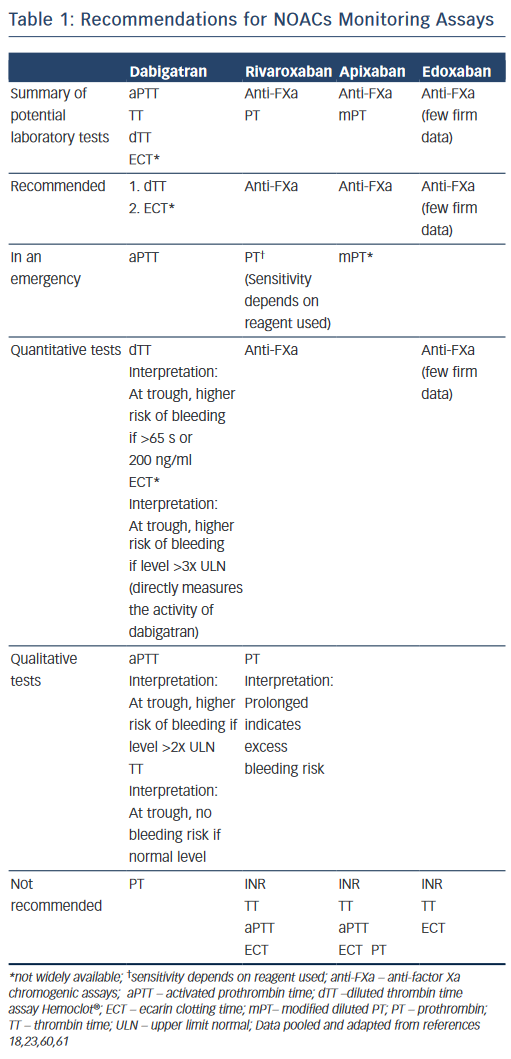
- For dabigatran, the dTT and ecarin-based assays are preferred and may be used for drug concentration measurements.
- For rivaroxaban and apixaban, anti-FXa activity is preferred for drug concentration measurements.
- For rivaroxaban alone, PT is more sensitive than aPTT; however, it should not be used for drug concentration measurements.
- For apixaban alone, both PT and aPTT are insensitive.
Recommendations for NOACs monitoring assays are summarised in Table 1.
Strategies for NOAC-related Bleeding and Reversal of NOACs Anticoagulant Effects
NOACs pharmacokinetics/pharmacodynamics are important when challenged with a patient who may be bleeding in the setting of NOACs exposure. Table 2 shows the clinical pharmacology and indications of the NOACs; however, an in-depth discussion of their pharmacokinetics/pharmacodynamics is beyond the scope of this review.
In view of the lack of readily available or reliable specific antidotes for the NOACs, institutional guidelines have provided general guidance to assist bleeding patients.18,23 These recommendations are often influenced by 1) the severity of the bleeding; 2) the pharmacology of the specific agent; 3) overdosing; 4) reversing the NOAC prior to an emergency surgery; and 5) renal function of the individual.
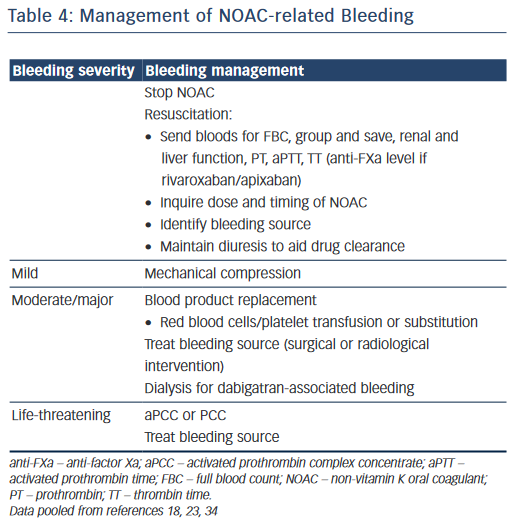
The cornerstones in managing NOAC-related bleeding include: 1) withholding the specific NOAC; 2) resuscitation (e.g. intravenous access, mechanical compression, fluid administration, blood product transfusion, maintaining diuresis to clear drug); 3) proceduralist-led interventions and 4) consideration for non-specific procoagulant agents given the lack of specific reversal agents.34
Non-specific reversal agents (e.g. prothrombin complex concentrates, activated prothrombin complex concentrates, recombinant factor VIIa [rFVIIa]) are considered for reversal of NOACS, however, there is no high-quality evidence to support their use. The use of these agents is also associated with small risk of thrombosis and it is recommended that they are reserved for severe and life-threatening bleeds.
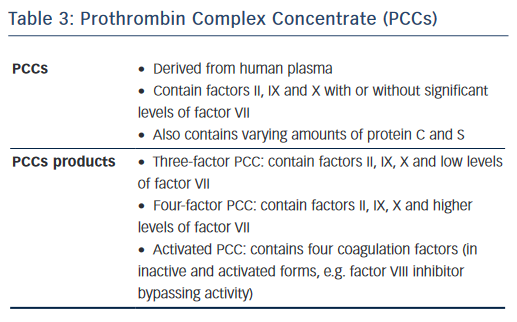
Prothrombin Complex Concentrates (PCCs) contain vitamin K-dependent coagulation factors II, IX, X, varying amounts of factor VII and proteins C and S. Three-factor PCCs contain factors II, IX, X and low levels of factor VII; while four-factor PCCs contain factors II, IX, X and higher levels of factor VII (see Table 3). PCCs have been shown to reduce bleeding in animal models with varying degree of success on haemostatic parameters.35–39 In human studies involving healthy volunteers, PCCs normalised PT in those receiving rivaroxaban.40–42 However, PCCs did not correct the aPTT, ECT or TT induced by dabigatran although PCCs enhanced the rate of thrombin generation.40,41,43
Activated Prothrombin Complex Concentrate (aPCC) contains factors II, VII, IX and X. In animal models, aPCC corrected the anticoagulant effect of high-dose rivaroxaban.44,45 Siegal et al have reviewed that in in vitro studies aPCC corrects some abnormal clot-based coagulation tests, thromboelastometry parameters and thrombin generation indices induced by dabigatran, rivaroxaban and apixaban.34
rFVIIa. In in vitro studies, rFVIIa demonstrated variable effects on abnormal clot-based coagulation tests, thromboelastometry parameters and thrombin-generation indices induced by rivaroxaban and apixaban.34
Antifibrinolytic Agents. Tranexamic acid interferes with fibrinolysis thus stabilisng fibrin clots. However, its prothrombic potential in NOAC-associated bleeding is unknown.
Haemodialysis. Dabigatran may be removed from the circulation by haemodialysis in patients with major bleeding or surgical procedures. This approach takes 4–6 hours, and is more desirable in patients with end-stage renal disease and overdosing.46
Expert Opinion: What Should We Use?23,34
- It is important to inquire about the exact time of last NOAC intake.
- Based on limited clinical data, PCC and aPCC can be administered in severe/life-threatening bleeding.
- Haemodialysis may be useful for dabigatran removal.
- Tranexamic acid may also be added.
Table 4 shows a suggested algorithm for managing NOAC-related bleeding.
Significance of Antidotes for the NOACs
Much promising research into antidotes for the NOACs is underway.47,48 Gomez-Outes et al. have reviewed recent antidotes patents and their ongoing clinical trials.49 Specific reversal agents that are being investigated as direct NOAC antidotes are summarised below.
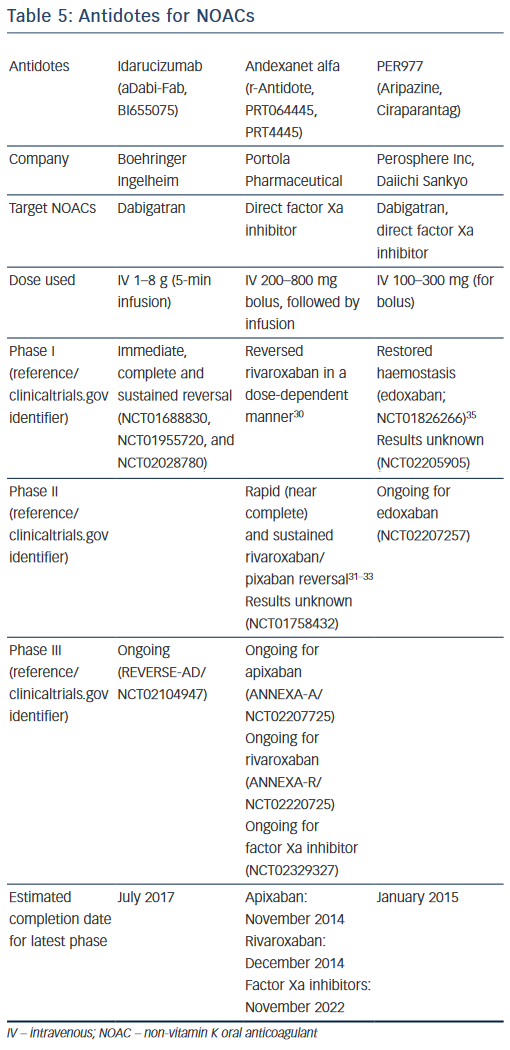
Idarucizumab (aDabi-Fab, BI655075)
Idarucizumab is a humanised monoclonal antibody fragment, or Fab, against dabigatran and its metabolites. It is generated from mouse monoclonal antibody, then humanised and reduced to a Fab fragment. Its structure is similar to thrombin, but with an affinity for dabigatran that is approximately 350 times higher than its affinity for thrombin.48 Dabigatran almost completely inhibits fibrinopeptide A (FPA) formation at the wound site, and idarucizumab is aimed at restoring systemic blood coagulation and re-enabling the formation of this fibrin.50 There have been at least three Phase I studies in healthy younger volunteers (clinicaltrials.gov identifiers NCT01688830, NCT01955720 and NCT02028780), which have demonstrated immediate, complete and sustained reversal of dabigatran-induced anticoagulation by idarucizumab. These randomised, double-blind, placebo-controlled studies showed that idarucizumab effectively restored wound-site formation of FPA, and did not cause any clinically relevant side-effects, no pro-thrombotic effect and no return of anticoagulant activity.
Another important study has recently followed these three trials. It involved older volunteers/patients with mild or moderate renal impairment and demonstrated that a 5-minute infusion of idarucizumab was able to reverse the blood-thinning effects of dabigatran.51
Currently, a global Phase III study of patients on dabigatran with major bleeding or needing emergency surgery, is underway (REVERSE-AD, NCT02104947). The study is open to eligible patients in more than 35 countries, expected to enrol 250 subjects and due to complete July 2017. The primary outcome of REVERSE-AD is the maximum reversal of dabigatran by intravenous administration of 5 g idarucizumab, based on dTT and ECT, at any time point from the end of the first infusion up to 4 hours after the last infusion.
Andexanet Alfa (r-Antidote,PRT064445, PRT4445)
Developed by Portola, andexanet alfa is a universal antidote for factor Xa inhibitors. It is a small (39 kDa), catalytically inactive, human recombinant modified molecule that is similar to native factor Xa. Acting as a decoy receptor, it binds and sequesters direct factor Xa inhibitors, preventing them from inhibiting the activity of the native factor Xa, thus restoring normal haemostatic processes.47 In preclinical studies, a rivaroxaban-treated rabbit liver laceration model has shown andexanet alfa to restore haemostasis by sequestering the factor Xa inhibitor in a 1:1 molar ratio, reducing free fraction and anti-factor Xa activity of the factor Xa-inhibitor in plasma.47
A Phase I, first-in-man, randomised, double-blind, single-ascending dose safety and tolerability study conducted in 32 healthy volunteers has demonstrated that andexanet alfa reversed anti-FXa activity of rivaroxaban, with no thrombotic events or deaths reported.52
Phase II proof-of-concept double-blind, placebo-controlled studies in healthy subjects examined the reversal of NOACs by andexanet alfa. A bolus of andexanet alfa 210–420 mg for rivaroxaban and apixaban, and 600–800 mg for edoxaban provided a rapid decreased (near complete) anticoagulant activity dose dependently. Continuation of the infusion regimen resulted in a sustained reversal of rivaroxaban, apixaban and edoxaban that could achieve complete reversal dose dependently. Andexanet alfa was well tolerated, with no thrombotic events or antibodies to factor Xa or factor X observed.53–55
At least three Phase III randomised, double-blind, placebo-controlled studies in older subjects are ongoing to assess safety, efficacy and the reversal of rivaroxaban (NCT02220725), apixaban (NCT02207725) and factor Xa inhibitors (NCT02329327) rapidly after an intravenous bolus plus sustaining that effect through a continuous infusion. The latter is estimated to complete in 2022.
PER977 (Ciraparantag, Aripazine)
Developed by Perosphere, this is a small (500 Da), synthetic, water soluble, thermally stable, cationic D-arginine compound that has broad activity against various old (heparin, LMWH) and newer oral anticoagulants (dabigatran, rivaroxaban, apixaban, edoxaban). For patients taking NOACs, PER977 re-establishes the normal blood coagulation state by directly binding to the factor Xa and IIa inhibitors through non-covalent hydrogen bonding. It has no pro-coagulant properties and does not bind to human plasma coagulation factors or albumin. Preclinical in vivo animal models (rat-tail injury model) have shown PER977 to reverse dabigatran, rivaroxaban and apixaban, as confirmed by >90% reduction in blood loss in bleeding model.56
In human blood ex vivo, PER977 completely reverses rivaroxaban and apixaban in a dose-dependent fashion, as confirmed by aPTT and Xa assays.56
In November 2014, Perosphere published the results of first-in-human, 80-person, Phase I/II study.57 A single intravenous dose of PER977 (100–300 mg) was given 3 hours after edoxaban, and this restored blood clotting time to <10% above baseline within 10 minutes of administration, in comparison with placebo (12–15 h). These effects lasted for at least 24 hours. PER977 is currently in Phase II trials of patient receiving chronic edoxaban therapy. The advantages of PER977 compared with its rivals are that it is stable at room temperature and reverses the activity of all approved NOACs.
Table 5 summarises the antidotes for NOACs, currently in development. Gomez-Outes et al has also highlighted potential antidote reactions,which include hypersensitivity reactions (e.g. pyrexia), rebound anticoagulation and rebound hypercoagulation. To avoid rebound anticoagulation, the antidotes such as andexanet alfa is being administered as initial bolus injection followed by continuous infusion.49 Rebound hypercoagulation is the increase in thrombotic effect following cessation of antithrombotic medications, which has been previously observed after cessation of heparin and some thrombin inhibitors.58,59 Further studies will be needed to clarify these issues.
Conclusion
NOACs are a new class of anticoagulants that have pharmacokinetic and pharmacodynamic advantages over warfarin. Their attractiveness is translated clinically into the greater convenience of no laboratory anticoagulation monitoring. However, as all anticoagulants can potentially cause bleeding, having access to laboratory assays is important to facilitate some clinical management decisions. From a clinical perspective, what is needed is a simple, rapid, reliable and global test that reflects and quantifies the anticoagulant effects of NOACs.
The ideal antidote for NOACs would be a rapid universal with longer shelf-life, as it is unknown how often the use of an antidote is necessary in clinical practice. Specific antidotes for NOACs are not yet approved, although their development is at a fairly advanced stage. The development of specific antidotes to NOACs show promising results in neutralising the drugs.