Advanced catheter-based technologies employed for the ablation of symptomatic atrial fibrillation (AF) have revolutionised the management of this common sustained arrhythmia. In the late 1990s, premature depolarisations originating from the myocardial sleeves within the pulmonary veins were recognised to initiate AF. This landmark discovery rendered pulmonary vein isolation (PVI) as the cornerstone of the nonpharmacological management of AF.1 The utilisation of modern 3D electroanatomical mapping and PVI-based ablation approaches in carefully selected patients with AF often offers therapeutic benefit.2 However, PVI alone may be insufficient, especially in patients with long-standing persistent AF. Linear ablation and ablation of complex fractionated atrial electrograms (CFAEs) have been proposed as strategies to improve the efficacy of PVI in patients with persistent AF with variable efficacy.3 The need to improve procedural outcomes while furthering the safety of the procedure has led to continuous efforts for the refinement of mapping and ablation technologies. Herein, we aim to review the current status of selected novel interventional strategies employed for the treatment of AF.
Focal Impulse and Rotor Modulation
One of the mechanistic hypotheses of AF suggests that the arrhythmia is initiated by localised triggers (drivers) and propagated in the form of high-frequency reentrant sources (rotors) or focal impulses that degenerate into fibrillatory waves.4 Targeting of such distinct AF drivers could therefore improve ablation outcomes. Two methods have recently been proposed for panoramic/global mapping of AF drivers:
- Intracardiac multielectrode contact mapping using a 64-electrode basket catheter,
- Non-invasive body surface potential mapping (BSPM); discussed in detail later in this review.
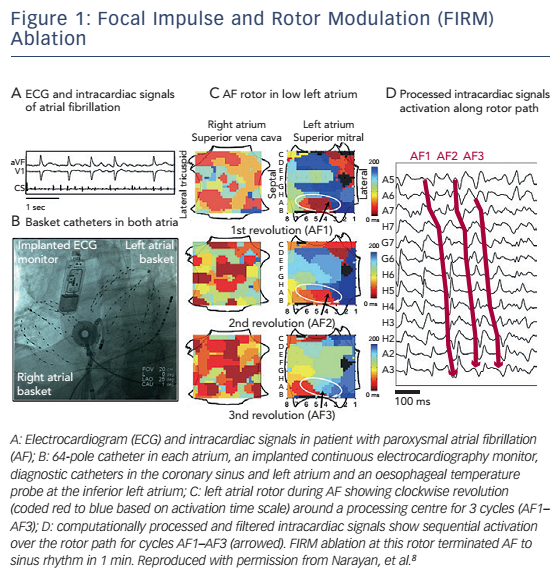
Both approaches utilise proprietary signal processing algorithms based on a number of steps which may include interpolation, filtering,frequency and phase domain analysis (Hilbert transformation and analysis of Shannon entropy).5 In the first approach, focal impulse and rotor modulation (FIRM) computational maps demonstrating rotors and focal impulses are created with the use of commercially available software (see Figure 1).6 The feasibility of this approach is based on the premise that focal sources are spatially and temporally stable and therefore may be feasible to be targeted for ablation. Interestingly, CFAE sites were shown to be in poor spatial association with AF sources.7 In the context of these observations, the pivotal prospective non-randomised Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation (CONFIRM) study was performed.8 Thirty-six patients (81 % with persistent AF) underwent FIRM-guided ablation and 71 patients (66 % with persistent AF) underwent conventional PVI. Localised AF sources were present in the vast majority of persistent AF cases (97 %) with a mean 2.1 ± 1.0 sources per subject. The number of rotors and focal impulses was higher among patients with persistent AF than those with paroxysmal AF (mean 2.2 ± 1.0 versus 1.7 ± 0.9, p=0.03). FIRM-guided ablation resulted in acute AF termination in 31 of 36 (86 %) patients, whereas conventional ablation was acutely successful in 13 of 65 (20 %) patients with sustained AF. After median 273 days, more patients in the FIRM-guided group were free of AF than in the FIRM-blinded group (82 % versus 45 %, p<0.001). In an extended 3-year followup of the CONFIRM study, FIRM-guided ablation conferred more sustained freedom from AF after 1.2 ± 0.4 procedures (78 % versus 39 % in the FIRM-blinded group, p=0.001) and a single procedure (p<0.001).9 Preliminary results from Precise Rotor Elimination Without Concomitant Pulmonary Vein Isolation For Subsequent Elimination Of PAF (PRECISE) trial performed by the same groups of investigators also demonstrated the superiority of the FIRM-guided over the FIRMblinded approach in paroxysmal AF.
These clinical trial results are promising; however, important limitations of the approach remain to be addressed. Electrogram quality is often insufficient for accurate mapping with the currently available multielectrode catheter technology, especially in atria with large dimensions.6 Due to insufficient electrogram quality, extensive interpolation may often be necessary which has its own inherent limitations. Additionally, while FIRM analysis generates maps that demonstrate stable rotors for several minutes, analysis of body surface potentials suggests that sites of high dominant frequency may actually be spatially and temporally unstable.10 Also, the mapping capability of the septal left atrium with this catheter is suboptimal while exclusively endocardial mapping may be unable to identify transmural or epicardial sources.11 Reproducibility of FIRM mapping in accurately identifying AF sources and superior clinical outcomes after FIRM-guided ablation will be critically important for widespread adoption of this novel technology.12,13
Body Surface Potential Mapping
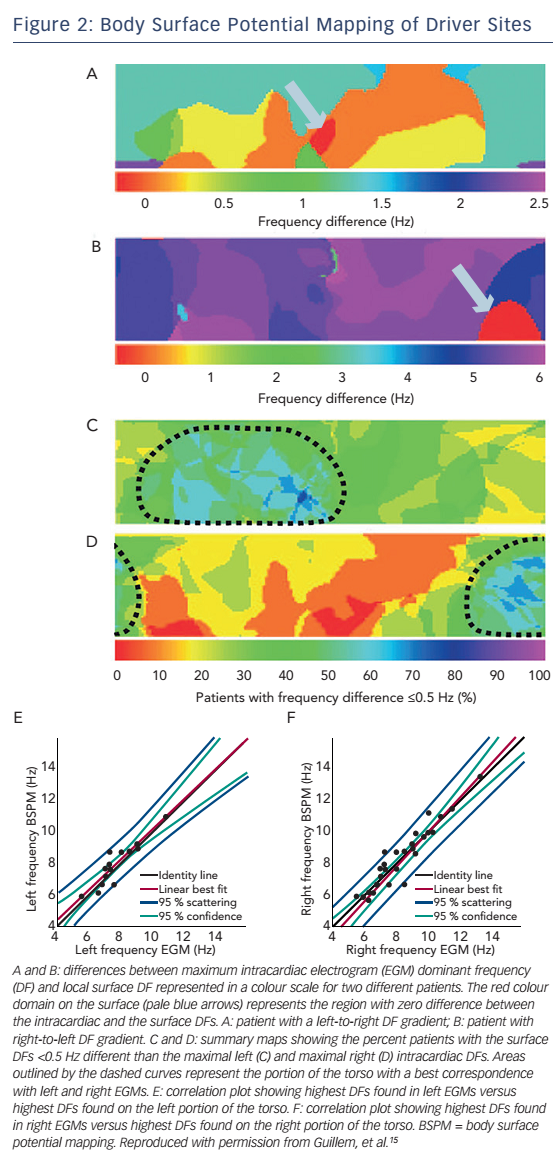
While three-dimensional electroanatomic mapping represents standard practice in AF ablation, pre-procedural non-invasive mapping may be useful in localising potential ablation targets. The standard 12-lead electrocardiogram is insufficient to characterise the complex electrical activation in AF. In a recent study, BSPM using 56 torso leads in addition to the standard limb leads was performed and four different patterns of wavefront propagation were described.14 In a subsequent study, the same group performed simultaneous BSPM and intracardiac real-time frequency electroanatomical mapping and observed good correlation between the highest dominant frequency sites in the right and left atria and the corresponding right- and left-sided surface leads (see Figure 2).15 Applying a similar concept of ‘panoramic mapping’, Haïssaguerre et al. integrated unipolar body surface potentials obtained from a 252-electrode vest with biatrial geometry obtained with high-resolution thoracic computed tomography (CT).16 Relative electrode positions were also determined by CT imaging, and activation, dominant frequency and cycle length maps were constructed. AF sources were also classified into focal and reentry (either functional or fixed-anatomical). In early clinical studies, the information obtained from this mapping approach was used to guide ablation. Whether non-invasive AF mapping can effectively guide AF ablation is a matter of ongoing investigation. Evidence is currently limited to a single-centre clinical study in 103 patients with persistent or long-lasting AF. Driverguided ablation resulted in termination of AF (into sinus rhythm or atrial tachycardia) in 75 % and 15 % of persistent and long-lasting AF cases, respectively.17 The preliminary results of another clinical trial, the multicentre Non-Invasive Mapping Before Ablation for Atrial Fibrillation (AFACART) study, showed overall similar success rates with driver ablation only.18 These studies demonstrated that AF drivers were rather unstable and had a mean duration of less than 1 second. However, there was a tendency of these drivers to occur at certain sites in the atria. Therefore, the investigators utilised maps that displayed the hierarchy of sites based on the probability of exhibiting a driver. Furthermore, approximately 80 % of the drivers were microreentrant and 20 % were focal.
Magnetic Resonance Imaging-guided Ablation
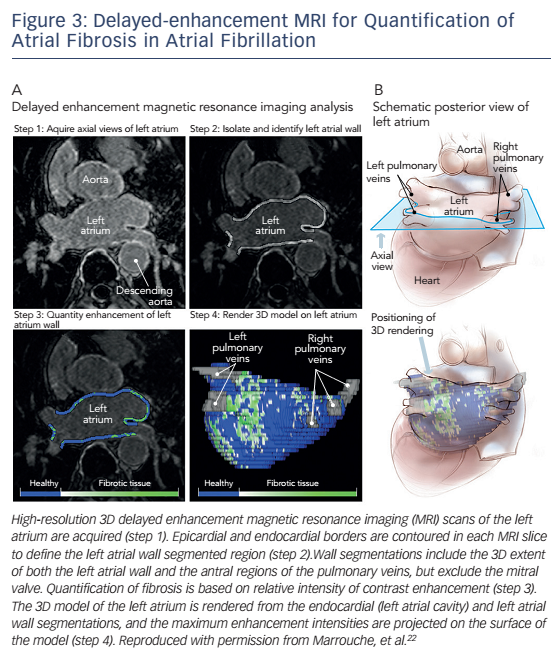
Atrial fibrosis, which plays an important role in the genesis and perpetuation of AF, can be detected by invasive electroanatomical mapping,19 but more recently delayed-enhancement cardiac magnetic resonance (DE-CMR) imaging has emerged as a promising noninvasive modality for the quantification and classification of the extent of fibrosis based on the Utah classification (see Figure 3).20,21 In the Delayed-Enhancement MRI Determinant of Successful Radiofrequency Catheter Ablation of Atrial Fibrillation (DECAAF) study, the degree of fibrosis was independently associated with arrhythmia recurrence after catheter ablation.22 Other prospective studies are currently underway where patients are being randomised to fibrosisguided versus conventional radiofrequency ablation. In addition to DE-CMR, T1 mapping is another MRI-based technique that has shown encouraging results in the pre-procedural planning of AF catheter ablation as it allows for direct signal quantification. In a controlled study of 112 patients undergoing radiofrequency ablation, the T1 time was the only predictor of 12-month arrhythmia recurrence in multivariate analysis.23
The real-time use of MRI to guide ablation intraprocedurally has also been investigated.24 Specifically for AF, using a 3-Tesla MRI system, Vergara et al. were the first to combine real-time MRI tracking of catheters with recording of electrograms in order to guide radiofrequency ablation and visualise lesion formation in a swine model.25 The same group used real-time DE-CMR to identify and target gaps in ablation lesions sets,26 even though it has also been argued that DE-CMR may not be accurate enough to reliably assess lesion distribution.27 While real-time MRI is radiation-free and allows for the accurate visualisation of the location and extend of lesion formation, its disadvantages may include the compatibility of catheters and existing ablation technology, cost and incompatibility with implantable cardiac devices or other hardware.
Ganglionated Plexi Ablation
Vagal denervation during circumferential PVI may improve the longterm outcome of ablation.28 The ganglionated plexi (GP), are located in the epicardial aspect of the junctions of all four pulmonary veins (PVs) with the left atrium and are responsible for both the sympathetic and parasympathetic innervation of the atrium.29 Since parasympathetic influences can facilitate AF by both increasing triggered activity and shortening the effective refractory period, it has been suggested that ablation of the GP may lead to more successful elimination of the arrhythmia. The approach to identify the exact location of the GP in the electrophysiology laboratory has evolved from the use of high-frequency stimulation (HFS) in order to elicit a vagal response30 to the anatomically-guided ablation of regions known to harbour the GP. The latter approach has been proven feasible and safe31 and in a direct comparison to HFS-driven GP ablation, it was also shown to be more effective.32
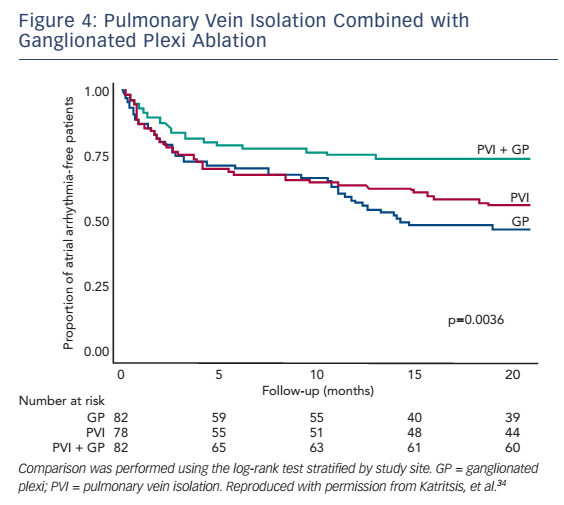
In clinical studies, GP ablation has been examined alone and in association with PVI in paroxysmal and persistent AF with variable results. In the first randomised controlled trial on the topic, Katritsis et al. compared GP ablation plus PVI with PVI alone in 67 patients with paroxysmal AF.33 At mean follow-up periods of 11.3 ± 1.9 months for the GP + PV group and 9.7 ± 3.4 months for the PV group, 15 (46 %) patients in the PV group and 25 (74 %) patients in the GP + PV group remained arrhythmia-free after a single procedure (log rank p=0.022). In a subsequent larger trial by the same investigators, 242 patients with paroxysmal AF were randomised to PVI alone (n=78), GP ablation alone (n=82), or PVI plus GP ablation (n=82).34 At 2-year follow-up, a total of 44 (56 %), 39 (48 %), and 61 (74 %) patients in the PVI, GP and PVI + GP ablation groups, respectively, remained in sinus rhythm (see Figure 4). These data support the role of autonomic denervation in the ablative management of AF. It is unclear, however, whether these outcomes are mediated by a truly independent effect of autonomic modulation or whether GP ablation simply results in more durable isolation of the pulmonary veins. Also, additional questions remain in regards to the safety of the approach. In theory, the application of extra radiofrequency lesions required for ablation of the GPs may increase the risks of tamponade and oesophageal injury, whereas excess atrial scar tissue formation could be pro-arrhythmic. Notably, however, in the aforementioned randomised trial of 240 patients, the only complication was one case of tamponade (in the PVI group) and the incidence of post-ablation atrial flutter did not differ between groups.34
Novel Ablation Technologies
Cryoballoon Ablation
PVI is an effective approach for the ablation of AF but its success depends heavily on the complete isolation of the pulmonary veins. This requires very meticulous point-by-point delivery of lesions for which radiofrequency energy source is used. The cryoballoon ablation technology, on the other hand, delivers a single circumferential isolation lesion with the use of a catheter-guided cryotherapy balloon. Advantages include the lower risk of certain complications, such as pulmonary vein stenosis and atrio-oesophageal fistulae, and the significantly reduced procedural time. Additionally, cryolesions are more homogeneous and are considered less arrhythmogenic than RF lesions. The first cryoballoon ablation system received Food and Drug Administration (FDA) approval in the United States in 2010 and its use has been steadily growing. The pivotal Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP-AF) trial (2001–5) showed that PVI with the first-generation cryoballoon effectively eliminated paroxysmal AF in 82 % of patients during a mean follow-up of ~3 years. The overall complication rate was 4 % in that trial.35 A metaanalysis of 23 randomised and observational studies showed acute procedural success rate of almost 100 % among patients achieving complete PVI with the first-generation cryoballoon system and 1-year single-procedure success of 60 % off any antiarrhythmic drugs.36 Acute and long-term efficacy results appear to be even more promising with the second-generation cryoballoon system, which is characterised by more homogeneous balloon temperature.37,38 This may, however, be at the expense of higher rates of complications such as phrenic nerve palsy and oesophageal thermal injury.37,39
The comparative effectiveness and safety of cryoballoon and radiofrequency ablation are also of great interest. The recently published FreezeAF randomised trial indicated the non-inferiority of cryoballoon ablation with regards to 1-year recurrence-free rates, but procedural complications, in particular phrenic nerve palsy, were more common with cryoballoon ablation.40 Further insights into the cryoballoon versus radiofrequency ablation comparison are expected from the ongoing FIRE AND ICE trial.41
Visually-guided Laser Balloon Ablation
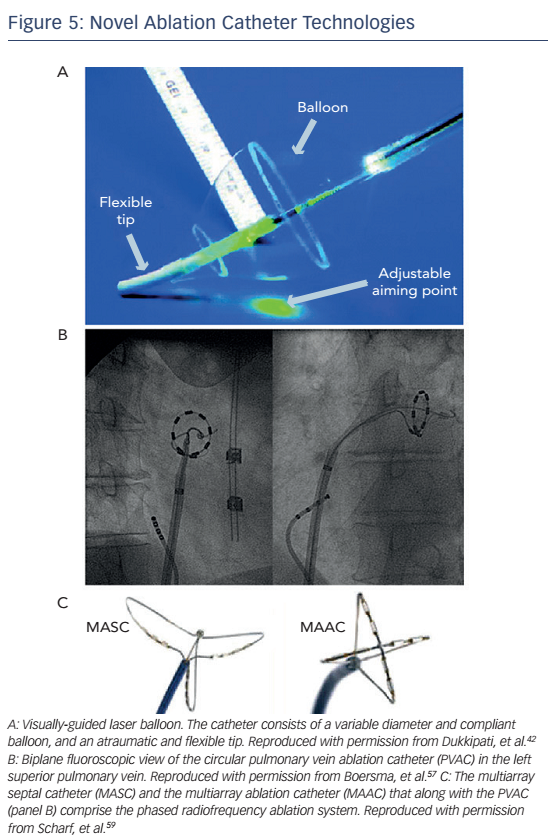
The visually-guided laser balloon (VGLB) ablation catheter (HeartLight®, CardioFocus Inc) is the only catheter that allows direct visualisation of the target myocardium to be ablated which may in theory improve the rates of complete isolation and eventually decrease AF recurrences (see Figure 5A). Similar to the cryoballoon catheter, it can deliver more homogeneous circumferential isolation compared with the pointby-point radiofrequency ablation. The system consists of a variablediameter compliant balloon with a central shaft through which a 2 Fr endoscope allows real-time visualisation of target tissue. Ablation is performed with a manoeuvrable 30° light arc originating from the central shaft that can be targeted to any location along the surface of the balloon to deliver laser energy (980 nm). Isolation of the PV is then confirmed with the use of a different circular mapping catheter. While the system is currently used in clinical practice in Europe, it is not yet FDA-approved in the US.
The first clinical study of the current generation VGLB system in 27 patients with paroxysmal AF was published in 2010 and demonstrated the feasibility of the approach.42 In a subsequent study of 56 patients, acute and 3-month isolation was documented in 98 % and 86 % of pulmonary veins, respectively,43 while another study of similar size demonstrated an arrhythmia-free rate of 60 % one year after ablation.44 This was in accordance with a multicentre study of 200 patients with paroxysmal AF in which freedom from atrial arrhythmias after one to two procedures was 60 % at 1 year, which is comparable to RF ablation.45 Tamponade and phrenic nerve injury rates of 2 % and 2.5 %, respectively, were raised as potential safety concerns in that study and, of note, associations between the lack of operator experience and longer procedure, ablation and fluoroscopy times were also demonstrated.
Finally, the recently released results of the HeartLight US pivotal trial comparing VGLB versus radiofrequency ablation in 353 patients with paroxysmal AF demonstrated the non-inferiority of VGLB with regards to freedom from treatment failure at 12-month follow-up (61.1 % in the VGLB group versus 61.7 % in the radiofrequency ablation group). The overall rate of adverse events was also similar between the two groups but VGLB was associated with higher risk of diaphragmatic paralysis (3.5 % versus 0.6 %, p=0.05) and lower risk of pulmonary vein stenosis (0 % versus 2.9 %, p=0.03).46
Radiofrequency Hot Balloon Ablation
The radiofrequency hot balloon catheter (Hayama Arrhythmia Institute) is another balloon-based ablation system aiming to achieve transmural circumferential PVI. The most updated version of this system is composed of a 1.8 MHz RF generator, a 13 F deflectable guiding sheath, a two-lumen catheter shaft and a highly elastic and compliant 20 μm thick polyurethane balloon which is inflated from 26–33 mm in diameter with ionised contrast medium diluted with normal saline. RF energy is delivered between a coil electrode inside the balloon and four cutaneous electrodes on the patient’s back to induce capacitive-type heating of the balloon.47 The efficacy and safety of this system have been assessed in a proof-of-concept animal study48 and in three subsequent human studies in paroxysmal and persistent AF.47,49,50 n the largest of these studies, among 100 patients with drug resistant paroxysmal (n=63) or persistent (n=37) AF, 92 patients were free of AF without antiarrhythmic drugs at mean follow-up 11 months and there were no strokes or atrio-oesophageal fistulae, but there were three cases of asymptomatic PV stenosis50. In the most recent but smaller study of 30 patients, after a similar follow-up period, two thirds of patients were free of AF with a single procedure and there were no procedure-related short-term or long-term complications.47
Contact-force Sensing Catheters
PV reconnection is a common cause of AF recurrence after PVI.51 Therefore the delivery of effective radiofrequency lesions that are likely to lead to permanent PVI is of paramount importance. In addition to the duration of lesion application, the contact force (CF) applied by the catheter tip on the myocardium is a major determinant of lesion size and depth. There is, however, a fine balance between too much CF that can lead to perforation and tamponade and too little CF that can lead to non-transmural or incomplete lesions. The need for better precision in CF application led to the development of two FDAapproved CF radiofrequency ablation catheters, which have become available in the last 2 years: ThermoCool® SmartTouch™ (Biosense Webster Inc) and TactiCath™ Quartz (St Jude Medical Inc). Using spring microdeformation or fibreoptic technologies, catheter tip direction and CF amplitude are sampled in rapid cycles of 50–100 msec and displayed in real-time.
Several, mostly small studies have examined the clinical utility of the CF sensing catheters. In the randomised study by Kimura et al., CF-guided ablation (n=19 patients) was associated with significantly reduced procedure time and residual conduction gaps compared with non-CF-guided ablation (n=19 patients).52 In the prospective non-randomised ThermoCool SmartTouch Catheter for the Treatment of Symptomatic Paroxysmal Atrial Fibrillation (SMART-AF) study, which led to the FDA approval of the ThermoCool SmartTouch catheter, effective use of CF monitoring (defined as remaining within the pre-selected CF range ≥80 % of the time during radiofrequency application) was associated with significantly higher success rate (probability of freedom from recurrence of 81 % versus 66 %).53 It should be noted, however, that in that trial tamponade complicated 2.5 % of the procedures which was higher than anticipated.54 More conclusive data on the utility of the CF sensing technology may be derived from the recently published TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR), in which 300 patients with paroxysmal AF were randomised to CF-guided ablation with the TactiCath Quartz catheter versus a standard radiofrequency ablation catheter.55 In this study, the non-inferiority of the CF catheter was proven, as the primary effectiveness endpoint was achieved in 68 % and 69 % of patients in the CF and control groups, respectively, while complication rates were similar. Efficacy was even higher when optimal CF was used (>90 % ablation with >10 g force). Results of all randomised and non-randomised studies were summarised in a recent meta-analysis that demonstrated a 37 % decrease of AF recurrences with use of CF sensing technologies.56
Multielectrode Ablation Catheters
Multielectrode ablation catheters were designed to overcome some of the limitations of point-by-point radiofrequency ablation, namely the potential for non-contiguous and/or non-transmural ablation lesions and the risk of injury of adjacent structures with extensive unipolar radiofrequency energy application. The pulmonary vein ablation catheter (PVAC, Medtronic Ablation Frontiers) is a circular, decapolar mapping and ablation catheter with a 25 mm diameter array at the distal tip with adjustable diameter allowing positioning in PVs of variable diameter (see Figure 5B). The GENius™ multichannel, duty-cycled RF generator generates either unipolar or bipolar current with a fixed duty-cycle by a phase difference between the channels. This system showed 100 % success in isolating 369 PVs in 98 patients with paroxysmal AF with mean procedural and fluoroscopy times 84 and 18 minutes, respectively; these times are significantly shorter compared with conventional PVI. Six-month freedom from AF off antiarrhythmic medications was 83 %.57 More recently, results of the early clinical experience with another circular ablation catheter (nMARQ™, Biosense Webster) in 39 patients with paroxysmal AF were released.58 The nMARQ catheter has 10 openly irrigated electrodes arranged on a circle with adjustable diameter. Mapping is performed with the same catheter using the Carto® 3 system (BiosenseWebster) and RF energy can be delivered in unipolar or bipolar fashion (though only unipolar RF was used in this study). An impedance-based technology built in the mapping system provides real-time information on electrode-tissue contact for optimal RF delivery. Successful acute isolation was documented in 99 % of PVs with mean procedure duration 86 minutes. Single procedure success rate was 66 % at a mean follow-up of 140 days. Only one (3 %) procedural complication was noted (tamponade not related to the ablation catheter) and there were no late complications.
In persistent AF, extensive substrate modification is required in addition to PVI, making the ablation procedure lengthy and complex. To increase procedural efficiency and efficacy, the PVAC catheter was combined with two additional catheters: the multiarray septal catheter, which contains three arms with six paired electrodes and is purposed for ablation of CFAEs in the septum, and the multiarray ablation catheter, which has four arms with eight paired electrodes and is purposed for LA mapping and ablation (see Figure 5C). Initial clinical results with this system were promising: in a multicentre study of 50 patients with persistent AF the 20-month success rate was 66 % (>80 % reduction in AF burden)59 and in a subsequent study of 89 patients 56 % were free of AF at 12 months after a mean 1.2 procedures.60 The Tailored Treatment of Persistent Atrial Fibrillation (TTOP-AF) study represents the only randomised evaluation of this ablation system to date.61 Patients with persistent AF were randomised to ablative management (n=138) or antiarrhythmic medications (n=72). The chronic effectiveness endpoint was met in 55.8 % patients in the ablation group (≥90 % reduction in cumulative AF and atrial flutter burden at 6 months) versus 26.4 % in the medical management group. Significant differences were also observed in quality of life and symptom severity in favour of the ablation group. However, the trial did not meet its predefined acute safety endpoint, an effect that was mainly due to the peri-procedural stroke rate of 2.9 % (4 of 138 patients undergoing ablation). This higher-thananticipated stroke rate was attributed to lack of operator experience and inadequate anticoagulation strategies in the study, in addition to intrinsic characteristics of the ablation system. While an increased risk of subclinical thromboembolic events with the PVAC catheter has been reported,62 other non-randomised studies using the multiarray ablation system have demonstrated clinical thromboembolic rates comparable to cryoballoon and irrigated RF ablation in mixed paroxysmal and persistent AF populations.63,64 On the basis of the TTOP-AF results, the Medtronic Ablation Frontiers system was not approved for use in the US; however, a large-scale real-world registry of the second-generation multiarray ablation system utilising the PVAC GOLD® catheter is currently underway in several European centres. In the PVAC GOLD catheter, the platinum electrodes are replaced by gold ones, which are associated with improved thermal efficiency. The second generation system is also considered less thrombogenic.
Hybrid Endocardial and Epicardial Ablation
Addition of epicardial ablation lesions to conventional endocardial lesion sets may increase the success rates of the ablation procedure. While the traditional open surgical ablation for AF developed by Cox is highly efficacious, it is an invasive and lengthy procedure.65 In contrast, a pericardioscopic radiofrequency-based approach allows for direct access to the epicardium for delivery of ablation lines without the complexity and morbidity associated with open surgical ablation, and importantly without the need for cardiopulmonary bypass. A combined endocardial and minimally invasive epicardial procedure (so called hybrid or convergent ablation) may offer superior results to each individual procedure alone, especially in patients with longstanding AF, by bridging gaps, achieving more complete PVI and eliminating also additional electrophysiological targets.66 It has also been suggested that sequential hybrid ablation may be superior to repeat catheter ablation in patients who have already failed one endocardial attempt.67 Clinical evidence on the efficacy and safety of the hybrid approach has emerged in recent years from small or moderate sized series of patients, but no randomised trials have yet been published assessing the comparative performance of this combined approach to catheter ablation only or surgical ablation only.68–73 In the largest published series to-date by Gehi et al., 101 patients with predominantly persistent or long-standing persistent AF (83 % of patients) underwent single-procedure hybrid ablation. Twelve-month arrhythmia-free survival was 66.3 % and 70.5 % with a single and with repeat ablation procedure, respectively.69 The success rate at 1 year (defined as sinus rhythm without antiarrhythmic therapy or repeat ablation) was higher (83 %) in another smaller series though the small sample size (n=26) limits the ability to draw reliable conclusions.70 Importantly, 6 (6 %) early post-operative major complications were documented in the Gehi et al. study, including two deaths (one due to atrio-oesophageal fistula and one sudden death without obvious aetiology identified). In a more recent series of the staged hybrid procedure in 50 patients, the incidence of major complications after the surgical phase reached 13.7 %, while there were no major complications after the catheter ablation phase.68 These rates may exceed the major complication rate of conventional catheter ablation for AF in the contemporary era.74
Conclusions and Future Directions
Continued innovation can help meet the urgent need to improve the outcomes of the nonpharmacological management of AF.75 The novel mapping and ablation technologies discussed in this review show great promise to that direction. Operator learning curve, costs and, most importantly, superior safety and effectiveness profiles compared with established strategies will be important for the widespread adoption of new technologies which should be tested and confirmed in multicentre prospective randomised trials.