The first session of Day 2 focused on the importance of the target atrial tissue as opposed to the ablation energy of the tool, because wall thickness and tissue composition play almost an important a role as the energy. Presentations described lesion efficacy and disease substrate, information derived from electrograms, and innovative approaches to speeding of ablation times.
Atrial Wall Thickness: The Missing Ingredient for Effective Tailored Ablation
Left atrial wall thickness varies with atrial location, pathological state and age. It is a potential early marker of adverse atrial remodelling and the atrial wall is the primary ablation target, making wall thickness a relevant consideration in RF ablation success, said Prof Mark O’Neill from London, UK, opening Day 2.
Based on the available literature, the thickness of the atrial wall may have a role to play in perpetuation of AF. In limited studies of patients with AF recurrence, the atrial wall has been demonstrated to be thicker in some zones than in patients without recurrence, and as the degree of fibrosis (as estimated by voltage) increases, so too does atrial wall thickness. In addition, many studies of RF lesion formation show that lesion depth is at least 3–4 mm, sometimes 4–6 mm at powers, contact force and durations typically employed for AF ablation.41–45 The atrial wall may be 2–3 mm thick, and this must be taken into consideration.
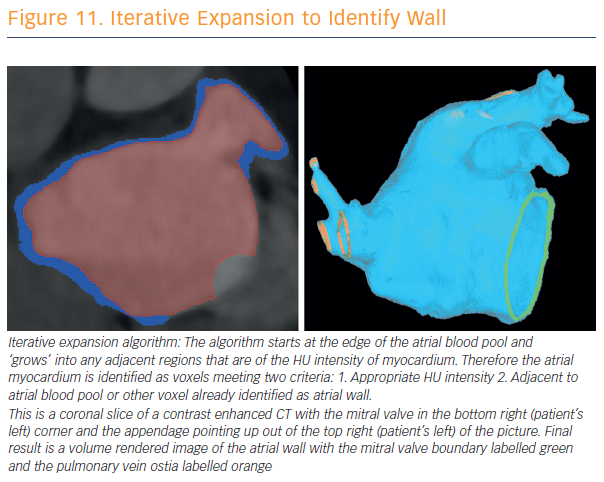
To assess and validate atrial wall thickness, 3D atrial wall thickness maps were created to inform catheter ablation procedures for AF (see Figure 11). Prof O’Neill and his team have validated their method for measurements in porcine hearts.46 CT is currently the best non-invasive modality for wall-thickness assessment, a calculation that may provide a reproducible means for assessment of disease progression. Awareness of local wall thickness is likely to encourage less rather than more ablation in most locations.
“We have been able to demonstrate that the atrial wall thickness pertaining to the appendage is greater at the anterior aspect of the appendage followed by the superior, inferior and posterior aspects,” said Prof O’Neill.
The sites of reconnection are most commonly at the thickest site, the anterior site, followed by the next thickest, and so on. Where there was acute reconnection, this was more commonly seen in thicker than thinner atrial sites. More work is needed before recommending ablation targets identified using MR or CT-derived wall thickness information. There’s also a new area to explore. It is possible that an increase in left atrial wall thickness may in fact be an early marker of propensity to AF – earlier than fibrosis detected on an MRI scan – and something that could be more widely applicable to early detection of patients at risk of AF.
Bipolar and Unipolar Electrogram Changes as In Vivo Marker for Transmural Lesion
Dr Agustín Bortone of Service de Cardiologie Hôpital Privé les Franciscaines in Nîmes, France, spoke on unipolar and bipolar electrogram changes as in vivo markers of transmurality creation. Several publications have shown that local electrogram amplitude reduction greater than 50 % is associated with the formation of transmural lesions (TL).47,48 However, the monitored bipolar (most commonly) electrogram from the tip to the first recording ring (tipring) extends beyond ablated tissues, reducing the effect of RF application on the monitored electrogram. In addition, other studies do not confirm the relationship between a halving of the electrogram amplitude and the creation of TL. Some show that achievement of split potentials rather than electrogram amplitude reduction is predictive of TL, while others argue that a signal amplitude reduction of 80% or even 90% is a marker of TL, rather than 50 %.49-51
Dr Bortone said that it seems logical to consider that the decrease in the amplitude of electrogram – either unipolar or bipolar – is correlated with the depth of the lesion and thus to TL achievement. However, with conventional catheters the electrogram analysed takes account of information that exceeds the treated tissue. There is a lack of specificity, and the percentage of exact reduction of the electrogram amplitude defining a TL is not clear.
Dr Bortone discussed and summarised the literature regarding these issues, and his analysis concluded that bipolar electrogram (BE) and unipolar electrogram amplitude reduction monitored by conventional catheters does not allow accurate TL creation assessment, in part because the perfect percentage reduction value is unknown. Bipolar electrogram amplitude reduction monitored by mini electrodes appears promising as a marker of TL creation. Unipolar electrogram morphologic changes to complete a positive signal are clear, reproducible and accurate markers of TL creation. BE morphologic changes are highly variable and, therefore, very difficult to use as real-time markers of TL creation.52 Transmurality is either functional and reversible, or corresponds to a necrotic and irreversible state.
In conclusion, the association of the monitored BE reduction (plateau) monitored by mini electrodes and the analysis of the morphologic changes of the unipolar electrogram may improve our ability to assess TL creation. The CF-sensing technology, as well as short-duration and high-power RF applications may increase the probability of creating transmural and necrotic (irreversible) lesions. The whole challenge lies in creating transmural, irreversible AND non-extramural lesions.
Can Short-duration and High-power RF Delivery be Safe and Effective?
Dr Elad Anter of Beth Israel Deaconess Medical Center in Boston, US, asked why the incidence of PV reconnection remains high despite adequate energy and tissue CF, and speculated that inadequate catheter stability, especially over a relatively long duration of 20 to 30 seconds may play a role by not necessarily creating tissue oedema. The answer may lie in higher energy for a shorter duration, which could provide more effective energy and, potentially reduced collateral injury.
Summarising numerous experiments in a thigh muscle preparation, Dr Anter said: “The combination of 90 W for 4 seconds at 10–20 g appeared to be effective and safe. These are the parameters that we have taken to the beating heart.”
He has focused on applying these findings using the QDot Micro Thermocool Smarttouch SF. Dr Anter has found that high power and short duration – 90 W and 4 seconds – of ablation can be effective in atrial tissue. It produces full-thickness, irreversible cellular damage that, in all PVs, including the left PV, are wider and more contiguous, compared to standard energy delivery. The safety of this technology requires real-time temperature monitoring, limited to 65˚C, plus an advanced irrigation technology. Collateral damage appears to be smaller compared to conventional ablation, probably due to reduced conductive heating.
Multimodality Assessment of the Atrial Fibrillation Substrate: Fat, Fractionation and LGE
Dr Saman Nazarian from the University of Pennsylvania, in Philadelphia, US, explored the impact of the substrate components. He noted that epicardial fat is associated with AF. Fat is metabolically active, releases inflammatory cytokines and adipokines into the adjacent myocardium, and exhibits rich innervation from ganglionated plexi in the proximity of the PV ostia.
He and his team studied the epicardial fat distribution and examined its association with electrogram properties.53 They noted that epicardial adipose tissue is very strongly associated with bipolar voltage, and it has a regional character, meaning that where there is epicardial adipose tissue overlying that region, the bipolar voltage amplitude is decreased. The geographic distribution of fractionated electrograms also has a strong association with overlying epicardial fat.
Fibrosis is also associated with AF. There are different modes of fibrosis: reactive interstitial fibrosis that separates muscle bundles, and reparative fibrosis (final) that replaces dead cardiomyocytes.54 The presence of fibrosis interferes with electrical continuity due to non-conductive patches, slows conduction due to longer paths, alters the balance of refractory and excitability properties, and may anchor re-entry. Furthermore, due to fibroblast electrical coupling with cardiomyocytes, fibrosis may promote ectopic activity.
“Surprisingly, right atrial fibrosis is also quite common; in fact, there’s more fibrosis in the right atrial appendage compared to the left atrial appendage. This brings into question the forgotten chamber when we’re ablating AF and the potential role of fibrosis in the right atrium,” Dr Nazarian commented.
He went on to explain his work in image-based arrhythmia substrate assessment, and its promise as a tool for arrhythmia management in the era of ‘precision medicine’.55,56 Late gadolinium enhancement (LGE) on CMR is associated with reduced voltage, decreased atrial function, and decreased conduction velocity. The association of AF persistence with AF recurrence post ablation is entirely mediated by the extent of fibrosis at baseline. However, LGE on CMR does not distinguish reactive fibrosis (i.e. potentially transient) versus replacement fibrosis, which is permanent.
“I wasn’t fully convinced of gadolinium imaging, but we started to see these beautiful linear lesions following ablation, that not only show ablation related scar but also highlight areas of pre-existing fibrosis,” said Dr Nazarian. “Atrial function also closely associates with fibrosis as measured by LGE. Passive atrial EF is closely associated with fibrosis based on our data. Active EF is just on the border of statistical significance. All strain parameters are also strongly associated with the extent of late gadolinium enhancement.” These associations have obvious implications for stroke risk stratification in the setting of AF.