Ventricular tachycardia (VT) is a significant cause of morbidity and mortality in patients with ischaemic and non-ischaemic cardiomyopathies (NICM).1 Invasive management of drug-refractory ventricular arrhythmias (VAs) was initially described in patients with healed MI. The procedures were performed by cardiac surgeons and primarily involved endocardial resection of the visible scar.2,3 In the modern era, catheter ablation has become the cornerstone treatment for patients with drug-refractory VAs given equivalent outcomes, relative safety and lower procedural morbidity compared to surgical resection. In a recent meta-analysis, ablation has been shown to reduce VT recurrence and shocks from implantable cardiac devices and hospitalisation.4 Despite technological advancements in catheter design and an improved ability to better localise abnormal substrates, putative circuits and the site where VAs originate, current technologies remain inadequate, and durable success may be elusive when the critical substrate is deep or located near to critical structures that are at risk of collateral damage. In this article, we review the available and potential future non-surgical investigational approaches for the treatment of VAs (Table 1).
Anatomical Substrates of Ventricular Arrhythmias
In most patients, the primary strategy of VT catheter ablation is based on the identification of critical components of reentry circuits and modification of abnormal substrate which can initiate reentry. VT ablation is generally more likely to be successful in patients with ischaemic cardiomyopathy (ICM) than in patients with NICM, but the long-term recurrence rate of VT is still higher than desired.5 The substrate for the reentry circuit of VT was thought to be predominantly sub-endocardial, based on the study of post-infarction patients. Although ablation in ICM was predominantly subendocardial, epicardial ablation was required in >30% of patients with NICM.5 In addition, the varying pathophysiology of VT originating from intramyocardial fibrosis and conduction delay have been identified in patients with NICM.6,7 Detailed 3D electroanatomic mapping (EAM) has shown that the 2D planar perspective is an oversimplification for tachycardia circuits. This is especially true for circuits located deep in the mid-myocardium and sub-epicardium which are more likely to support wavefronts traversing a 3D path between surfaces with potential for exits on either side. This was confirmed with careful epicardial and endocardial mapping showing evidence of gaps in the activation consistent with deep intramural substate. In a retrospective study of ICM (48%) and NICM (52%) which mapped 151 morphologies, only 17% were confined to one myocardial surface, while 65% demonstrated transmural propagation and 18% demonstrated focal mid-myocardial reentry.8 This creates significant challenges in lesion delivery and is an important cause of failure of catheter ablation for VT.
New Technologies for Ablation of VAs
Advances in ablation catheter technologies include the use of higher impedance irrigation, changes in the energy source used for ablation, and pre-ablation cardiac imaging which has been introduced to improve the efficacy of catheter ablation for VT.9,10
Use of Higher impedance Irrigants
Normal saline (NS) has an ionic charge and will conduct electricity with lower impedance compared to myocardial tissue. The presence of positive ions in irrigation solution attracts electrons away and simultaneously reduces current flow to tissue as well as measured impedance during radiofrequency (RF) delivery. Reduction in ionic concentration and charge density using half normal saline (HNS) or 5% dextrose (D5W) has been shown to produce larger lesions in preclinical studies through preferential current flow into myocardial tissue increasing current density, maximum temperature and depth of conductive heating.11,12
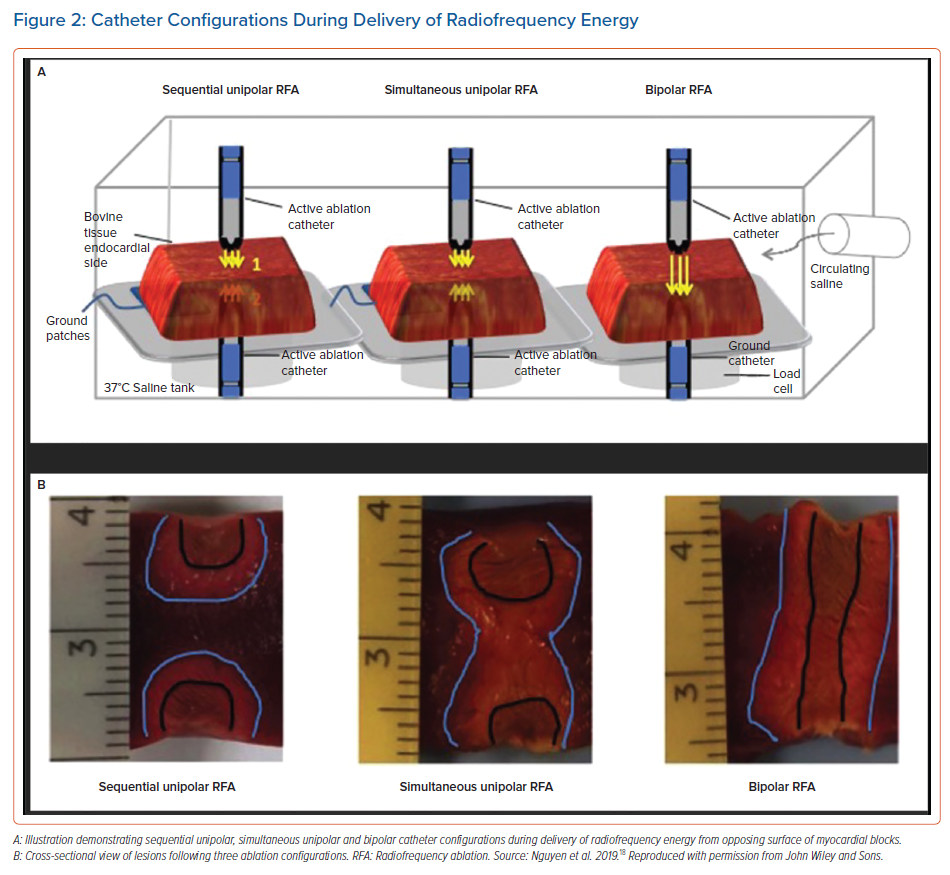
In an ex vivo study, unipolar RF ablation with HNS produced a larger lesion size in both endocardial and epicardial approaches compared to NS at the same power.11,12 In an in vivo study, HNS demonstrated larger lesion volume and greater impedance drops at similar power and contact force.12 In another ex vivo study, lesion depth and volume were higher with HNS and NS with larger lethal isotherm (Figure 1).11 The occurrence of steam pops (SPs) was also higher using HNS, with a high ablation index (>550) and longer lesion duration (>30 seconds) being associated with a higher chance of SPs.13
Clinical safety and effectiveness using HNS was studied in 95 patients with VT refractory to standard ablation resulting in an acute success rate of 83%.14 The interventricular septum or left ventricular summit area was the most common location (>50%), while papillary muscle (13%), the left ventricle free wall (15%) and the right ventricle (16%) accounted for the remainder. Long-term success defined as arrhythmia-free survival was 89.4%. While there were no significant complications attributed to HNS, SPs were noted by the operator in 12 of 94 cases. Low-volume irrigation (≤15 ml at power ≥30W) was the only variable associated with SPs. Given the risk of SPs, careful monitoring of impedance drops, an abrupt rise in temperature or impedance, and evidence of unusual echogenicity on intracardiac echocardiography are essential during ablation.
In summary, HNS irrigant is a reasonably safe and effective method to create deeper lesions. This might be indicated in patients refractory to standard ablation to target deeper intramural substrate. Lesion delivery with HNS is performed with power starting at 30–40 W and titrated carefully up to 50 W targeting 10–20% impedance reduction, paying close attention to temperature profile and impedance changes. While other advanced modalities require special tools and expertise, changing the irrigant only necessitates an adjustment in workflow. Experts suggest that augmentation of power delivery with hypo-osmolar irrigants should be reserved as a bailout strategy to reduce the risk of unnecessary complications.
Use of Simultaneous Unipolar Ablation and Bipolar Ablation
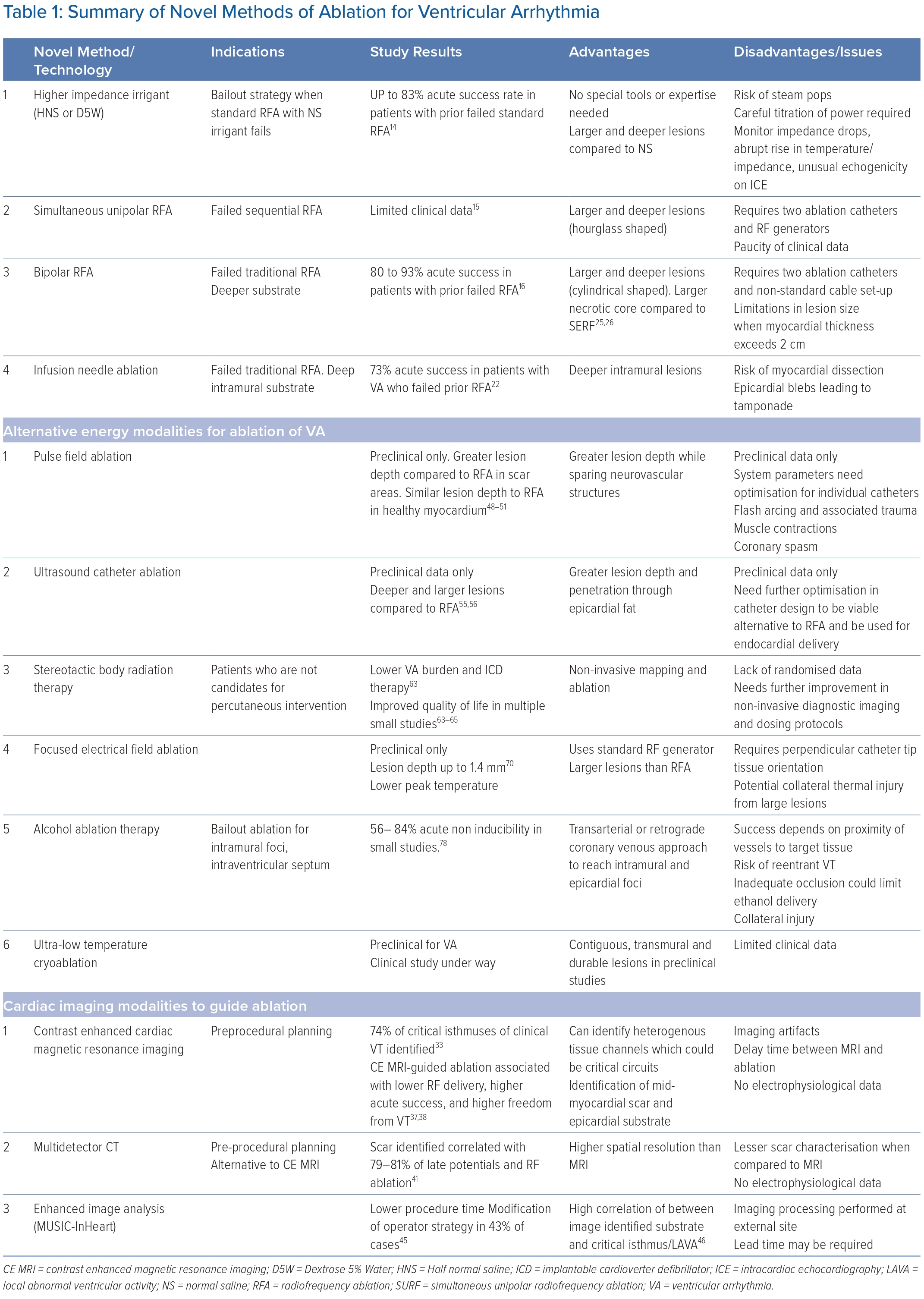
Simultaneous unipolar RF ablation is a technique that uses two ablation catheters connected to two different RF generators positioned on either side of the tissue to simultaneously deliver energy to achieve larger lesions. In a study of six patients with NICM and mid-myocardial substrate who failed to respond to prolonged sequential unipolar ablation, simultaneous unipolar RF ablation achieved acute success and long-term reduction in VT recurrence without procedural complications.15 Bipolar ablation was first described as a configuration for RF ablation and direct current ablation over three decades ago and has gained renewed interest for ablation of intramural substrate.16 Bipolar ablation involves the delivery of energy through two different ablation catheters placed on opposing surfaces of the targeted cardiac tissue. One catheter’s electrode serves as an active pole and the other is a grounding pole (Figure 2).
In an ex-vivo study, bipolar ablation was more effective in creating transmural lesions on the interventricular septum than unipolar ablation.17 Another study found the largest and deepest lesions were produced using irrigated catheters oriented perpendicular to tissue. There was a higher incidence of SPs (12%) which was not statistically significant.16 Myocardial thickness was a limiting factor with larger size and greater depth noted to an average of 15 mm but not at 20 mm. In another ex vivo study, bipolar ablation demonstrated greater lesion depth compared to simultaneous and sequential unipolar RF ablation (Figure 2) but no difference compared to simultaneous unipolar ablation.18 Bipolar ablation is off-label as it requires a modification of the cable provided by the manufacturer. Although simultaneous unipolar RF ablation doesn’t require modification of cables, a second generator is needed to power both catheters during energy delivery. Also, simultaneous RF ablation produces a more hourglass-shaped lesion compared to the cylindrical lesions created by bipolar ablation which may affect the ability to target mid-myocardial substrate.
In a recent study among 14 patients with failed previous RF ablation for VT/premature ventricular contractions (PVCs), 13 had acute success and non-inducibility following bipolar ablation while seven of the 10 patients had long-term freedom from VA.16 In another series of six patients with refractory VT, bipolar ablation was successful in terminating five of six septal VT and two of three cases of free-wall VT.19 In a five-patient study, four patients underwent successful bipolar ablation of refractory left ventricular summit PVCs from between the earliest endocardial activation site in the coronary venous system (CVS) and the adjacent endocardium.20 In a series of six patients, simultaneous unipolar RF ablation was effective in eliminating VT in patients with NICM and intra-septal VT refractory to conventional RF. Due to paucity of clinical data, larger ongoing clinical studies on the use of bipolar and simultaneous unipolar ablation may help elucidate ideal patient selection and provide data regarding acute as well as long-term success and safety.
Although evidence is limited and is still ongoing, case series and small studies suggest that bipolar ablation may be a viable approach in patients with challenging VT substrate. This would be especially indicated in patients with deep intramural substrate refractory to traditional RF ablation. Ablation is initiated at a power of 30–40 W and titrated carefully up to 50 W between the two ablation catheters targeting 10–20% impedance reduction, paying close attention to temperature profile and impedance changes. Complexity in the use, challenges in set-up, and the inability to address very thick substrate (>20 mm) limit its wider application. While visualisation of both catheters can be accomplished using commercially available equipment when using the EnSite electroanatomic mapping (EAM) system (Abbott), technology enabling visualisation of both catheters with the CARTO EAM system is only available to institutions participating in the investigation device exemption trial (NCT02374476).21
Use of Infusion Needle Ablation
Infusion needle ablation (INA) (Biosense Webster) uses a novel catheter with a 27 G extendable/retractable needle tip that can be extended into the myocardium up to 6–12 mm and produces lesions which extend deeper than the tip of the needle. RF ablation is applied in temperature-controlled mode (set to 60oC), with power initially limited to 15–35 W which is manually increased to achieve a temperature of 60oC.22 The needle is irrigated at 2 ml/min for the duration of RF ablation.
Stevenson et al. evaluated INA in 31 patients with left ventricle dysfunction who had failed at least one prior catheter ablation for sustained VT or non-sustained VT.22 INA demonstrated 73% acute suppression of VA and 48% of patients remained free of VA at 6 months. The majority of targeted substrate was in the septum and peri-aortic area. Although saline injection creates larger lesions, the study showed that it could cause dissection through myocardial tissue and result in epicardial blebs leading to tamponade. In another study of 119 patients involving 136 procedures with failed endocardial ablation, 57 patients underwent ablation with INA while the remaining underwent either epicardial ablation, simultaneous unipolar ablation, or trans-coronary ethanol infusion.23 INA did not demonstrate a significant difference in procedural characteristics or acute procedural success (about 50%). There was also no difference in long-term outcomes including mortality and VT recurrence at 6 months. In another study of 35 patients with PVC refractory to standard ablation, INA demonstrated 71% acute success and 73% success at 6 months.24
A second needle catheter, the saline-enhanced radiofrequency (SERF) catheter, is built with a 25 G needle which uses heated saline injected into the myocardium during RF delivery and demonstrated transmural lesions in a canine model.25 In a first-in-person study, 32 patients with drug-refractory VT were enrolled across six centres. Acute procedural success was 97% for suppression of inducible VT and subsequent use of device therapies were reduced by 89% on intermediate follow-up. However, complications (15.6%) were observed including two peri-procedural deaths due to embolic mesenteric infarction and cardiogenic shock, two strokes and pericardial effusion.26 Two clinical trials involving this promising new technology are actively enrolling subjects (NCT03628534; NCT0299446).
Use of Cardiac Imaging to Define Structural Arrhythmogenic Substrate to Guide Catheter Ablation
Catheter-based ablation of VA relies on EAM which can be time-consuming, suboptimal in delineation of intramural substrate and attenuated by epicardial fat. Multi-modality cardiac imaging can address some of the limitations of EAM. Nuclear imaging techniques using radiolabelled tracers have been used to identify scar regions and areas with active inflammation.27 Although useful for specific cardiomyopathies such as sarcoidosis, nuclear medicine techniques have largely been superseded by contrast-enhanced cardiac MRI (CE MRI) for imaging of the arrhythmogenic VT substrate. Multidetector CT (MDCT), CE MRI and intracardiac echocardiography can augment catheter-based ablation by providing detailed anatomical information, delineating myocardial scars and identifying epicardial fat.28 MDCT offers higher spatial resolution and CE MRI is able to identify myocardial fibrosis, which has made them popular modalities for preprocedural planning and image integration during ablation.
Contrast-enhanced Magnetic Resonance Imaging
CE MRI can differentiate normal myocardium from scarred myocardium by highlighting areas of fibrosis with a resolution of 1.4 x 1.4 mm with newer models having a spatial resolution of nearly 1 mm3.29 Using processing algorithms based on pixel signal intensities, CE MRI can differentiate dense scar and heterogenous scar (border zones) from healthy myocardium.28 Heterogenous tissue channels (HTC) are narrow pathways consisting of healthy tissue surrounded by scar or electrically non-excitable medium, such as fat or blood, connected to healthy myocardium located within areas of dense scarring.29–31 These HTCs identified by CE MRI correlated well with EAM in patients with post-infarction monomorphic VT.32 In another study of 21 post-infarction patients, CE MRI identified about 74% of the critical isthmus of clinical VT.33 CE MRI can be useful for preprocedural identification of heterogeneous scars and HTC to plan and guide targeted mapping thereby optimising procedural workflow. Accurate images from EAM are essential for using CE MRI images to guide ablation. In addition to automated algorithms, use of well-defined structures such as the aortic arch and pulmonary artery can minimise rotational errors.29
EAM with a bipolar voltage cut off <1.5 mV showed a good correlation with transmural scar but not with non-transmural heterogenous scar.29,34 Histological studies have also shown that EAM (bipolar and unipolar) did not demonstrate a good correlation when identifying fibrosis in patients with NICM.35 The importance of scar location was highlighted in another study, which showed just a 2 mm rim of viable endocardium overlying otherwise transmural scarring could result in a falsely normal bipolar voltage map, although a unipolar map can sometimes overcome this limitation.36 CE MRI can better identify a mid-myocardial scar which may be missed on the bipolar map.29 CE MRI can also help identify epicardial substrate which can then guide appropriate patient selection for the epicardial-endocardial ablation approach.29 MRI can also be used for post-ablation characterisation of the block of HTC channels although the clinical significance of this on long-term outcomes is unclear.
In a study of 123 patients with post-MI VT, use of cardiac imaging (CE MRI or MDCT) was an independent predictor of lower VT recurrence.37 In a prospective non-randomised study of 159 patients, Andreu et al. compared CE MRI-aided ablation (n=54) to standard ablation (n=105) for VT. CMR-guided scar dechannelling was associated with a lower need for RF delivery, higher non-inducibility rates after substrate ablation and higher freedom from VT.38 Increasing evidence has warranted updates to guidelines with recommendations for the use of CE MRI in patients with ischaemic and non-ischaemic cardiomyopathy to reduce VT recurrence (class 2a) and for preprocedural planning (class 2a).1
It is important to note certain limitations in image integration due to delayed time between imaging and ablation procedure.29 This may be overcome by acquiring images directly in the electrophysiology laboratory or as close to the procedure as possible. Although MRI can provide tissue characterisation identifying border zone and potential conduction channels it cannot provide electrophysiological information on conduction velocities, propagation and confirmation of VT circuits which would need to be corroborated functionally with EAM and entrainment manoeuvres. This may be overcome in the future with the use of surface ECG data integration to identify potential circuits. Imaging artefacts in patients with implantable cardioverter-defibrillators (ICDs) can limit resolution but may be minimised by using newer protocols and algorithms. Live MRI-guided chemoablation using MRI-conspicuous needles has been studied in a preclinical model but requires validation of feasibility in clinical studies.39
Multidetector Computed Tomography
Although MDCT is less capable of scar characterisation than CE MRI, it has a higher spatial resolution (0.5 mm) and can therefore improve the definition of the anatomy of complex cardiac structures such as papillary muscles. It can also identify sensitive extracardiac structures such as phrenic nerve, epicardial coronary arteries and fat. MDCT does show a good correlation with EAM for scar in patients with ICM (transmural scar) but not in patients with NICM (non-transmural scar). Although MDCT can identify conducting channels in ischaemic patients with transmural scar, when compared to CE MRI it fails to detect a significant proportion of the arrhythmogenic substrate. However, MDCT can still be a valuable alternative, especially when CE MRI is contraindicated, suboptimal or unavailable.29,37
The use of late iodine-enhanced MDCT in delineating scar that correlated with EAM as well as successful ablation site was initially demonstrated by Crean et al. in a patient with ICM and recurrent ICD shocks.40 In a subsequent study of 42 patients referred for VT ablation (35 with an ICD), Esposito et al. found good correlation between scar areas identified on CT with delayed enhancement and EAM.41 Late potentials and RF ablation points fell in segments of scar identified by MDCT in 79% and 81% of the cases, respectively. Karimianpour et al. recently reported on 192 consecutive patients with a history of coronary artery bypass graft (CABG) scheduled to undergo epicardial VT ablation. Prior to ablation, they employed a novel pre-ablation CT imaging method to assess wall thinning and scar localisation using late iodine enhancement. In the protocol, 0.6 g/kg body weight of iodine was injected followed by dual-energy CT image acquisition 7–10 minutes after contrast injection. After post-acquisition processing, 3D models indicating locations of highly dense and less dense scars were constructed and then integrated with the 3D EAM system at the time of catheter ablation. In the cohort, 81% of CVS target sites correlated with scars observed using late iodine enhancement on CT.42
Enhanced Image Analysis and Processing for Substrate Identification
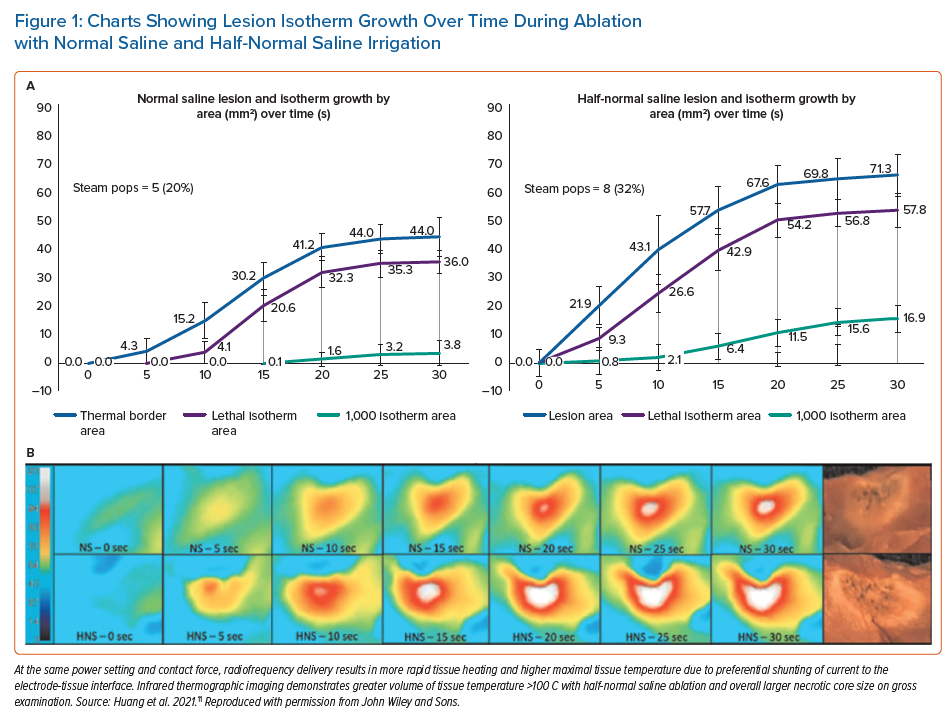
A proprietary software Multi-modality Platform for Specific Imaging in Cardiology (MUSIC) has been studied for its use in guiding scar-related VT ablation. An international consortium of sites using MUSIC/inHEART technology for VT ablation is actively examining its benefit on acute and long-term procedural outcomes with RF and other treatment modalities.43,44 In a prospective two-centre study examining the procedural impact of MUSIC on 42 patients who underwent VT ablation, the investigators found that VT non-inducibility and VT-free survival was similar between MRI/MDCT and EAM-limited groups, but procedure time was lower in the MUSIC-guided MRI/MDCT group (151 ± 33 versus 180 ± 53 minutes, p=0.01).45 Additionally, a single-arm retrospective study of a mixed population of 116 patients with VT (67 ischaemic CM, 30 NICM, 19 arrhythmogenic right ventricular cardiomyopathy) who underwent ablation procedures guided by MDCT/MRI with MUSIC image processing demonstrated a high correlation between image identified substrate and critical isthmuses and local abnormal ventricular activity (LAVA).46 In this study, image integration led to modification of operator ablation strategy in 43% of cases (Figure 3). In another study of 35 patients with VT and a history of CABG, MUSIC and MDCT was used to determine mid-myocardial substrate in patients undergoing epicardial ablation via the CVS.42
Alternative Energy Modalities on Ablation of Ventricular Arrhythmias
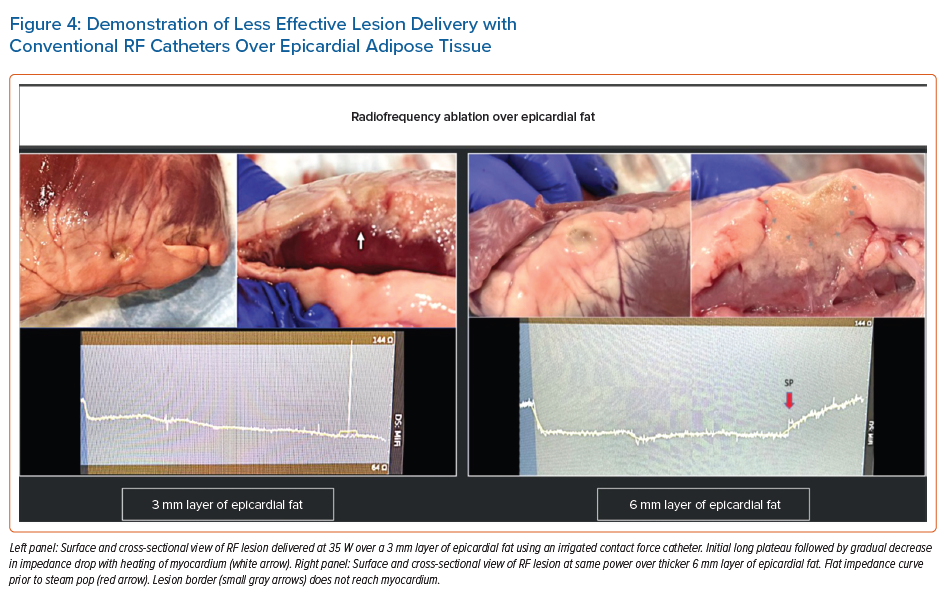
RF ablation has been the dominant energy source modality for treatment of VAs. However, energy penetrance and ultimately lesion size are significantly affected by tissue composition with reduced penetrance in dense scar, across coronary cusp tissue, CVS and epicardial adipose tissue (Figure 4). The risk of thermal-related collateral injury also poses a challenge for operators.
Use of Pulsed-field Ablation (Preclinical Only)
Pulsed-field ablation (PFA) is under investigation as a novel technique for treatment of VAs in preclinical studies. It is a mostly non-thermal modality of ablation relying instead on delivery of ultra-short, high-voltage electrical impulses to irreversibly electroporate cell membranes through the formation of nanopores leading to the derangement of cellular structures necessary for metabolic function. Since cardiac myocytes may have a lower threshold for electroporation than other tissues, operators can in theory selectively target the myocardium while sparing collateral structures.47 For ventricular applications, PFA catheters using a proprietary sequence of biphasic pulses lasting micro- or nanoseconds delivered in a bipolar fashion between the electrodes have been studied.47 In a proof-of-concept in vivo study, the left and right ventricles of four healthy pigs were successfully and safely ablated using PFA.48 Remapping after 35 days demonstrated the persistence of low voltage at the areas of ablation. Necropsy histology demonstrated complete and homogenous fibrosis within the lesion with no thrombus and a narrow zone of myocytolysis along the lesion border. Lesion depths of up to 9.4 mm and widths up to 28.6 mm were observed. A higher amount of energy delivered caused larger lesions. Subsequently, Im et al. compared PFA and RF ablation in an in vivo study of 10 pigs with healthy and post-infarct left ventricular myocardium.49 PFA (biphasic, µsec, scale pulse, 1800–2000 V, <10 second) demonstrated greater lesion depth compared to RF ablation (up to 60 seconds, 35–50 W, >10-ohm impedance drops) in areas of scar, but lesion depth was similar to RF ablation in healthy myocardium while sparing neurovascular structures. There was no difference between linear and basket PFA catheters in terms of lesion depth.
PFA of the interventricular septum (IVS) has also been examined in animal models. Tan et al. tested the ability to deliver through active fixation pacemaker leads in five canine models to target mid-myocardial substrate.50 They found the procedure to be safe and feasible using a double pacemaker lead configuration (bipolar, higher energy delivery with nanosecond pulses) creating a durable reduction in signal amplitude at the time of procedure and confirmed on follow-up with CE MRI and histopathology. In a recent study, van Zyl et al. demonstrated the feasibility of bipolar ablation from both sides of the IVS using solid tip catheters with pulse trains delivered as micro- and nanosecond bursts.51 Although acute atrioventricular block and left ventricular dysfunction was observed, both resolved on chronic evaluation except for persistent right bundle branch block in 38% of subjects. Cardiac MRI and histology demonstrated deep lesions with depths of 2.6 ± 2.1 mm.
There are potential limitations of PFA. While the electroporation effect of PFA is non-thermal, it still generates heat due to the delivery of electric fields through resistive tissue, especially at higher energy. Optimisation of system parameters such as waveforms, frequency and pulse duration, among others, has been shown to minimise this occurrence. Another limitation of simply increasing current densities to achieve depth is the risk of flash arcing at the electrode surface which may generate a vapour globe with release of intracellular plasma and cause unintended injury to nearby structures from barotrauma akin to direct current ablation performed in the past. High-energy monophasic deliveries may be particularly prone to this phenomenon and can also cause muscle contractions which could affect catheter stability and patient comfort. Smaller electrode surface area during PFA delivery may have higher proclivity for flash arcing phenomenon and hence both catheter design and applied waveform will be critical.
The long-term effects of clinically effective doses of PFA on major coronary vasculature remain unknown. While a preclinical study suggested relative preservation of the architecture of nearby micro vessels after PFA, transient ST elevations may be observed after PFA, and tunica media fibrosis and intimal hyperplasia of arterial vasculature have been reported by Koruth et al. on histological examination.52 Further, Cochet et al. demonstrated late gadolinium enhancement in the adjacent aorta after PFA delivery to the posterior wall of the left atrium in an MRI study. Major coronary vessel spasm from PFA applications has been demonstrated during epicardial applications in animal models and reported in the clinical setting.53,54 It is unclear if patients who develop coronary spasms are responsive to nitrates or other vasodilatory agents.
In summary, PFA represents an intriguing future alternative for ablation of VAs with the potential to deliver deeper lesions which may be relatively less affected by tissue substrate types than conventional modalities constrained by thermodynamic limitations during energy delivery. PFA may also be achieved with a shorter ablation time and may be associated with a smaller risk of collateral injury, but much remains to be elucidated on this front. Recently available preliminary data presented at major scientific meetings suggests that electrode contact is required for maximal and thus durable lesions. Therefore, catheter designs will still need to accommodate the optimisation of electrode-tissue coupling to maximise the electrolytic effects of PFA. Current data is still preclinical and future clinical trials evaluating PFA in patients with VT are required to evaluate its comparative efficacy and safety. It is likely that the viability of PFA for the treatment of VAs and its widespread adoption will rely on more than feasibility alone and will depend on its comparative benefit over established modalities such as RF.
Ultrasound Catheter Ablation (Preclinical)
Ultrasound catheter ablation is another modality intended to overcome the limitations of lesion depth in RFA. High-intensity ultrasound (HIU) involves applying ultrasound waves (1–10 MHz) at high amplitude (about 10 MPa) to generate localised thermal heating and necrosis. Ultrasound histotripsy is a method of tissue destruction through ultrasound cavitation by using pulsed wave ultrasound with high acoustic pressure amplitudes. Nazer et al. performed an in vivo study on pigs to compare HIU (15–30 W for 60 seconds) to RF ablation (25 W titrated up to achieve 10-Ω impedance drop) through an epicardial approach.55 They demonstrated that HIU generates deeper and larger lesions than RF ablation penetrating through epicardial fat as well as coronary arteries with greater epicardial sparing. Another in vivo study found that HIU at 6.5 MHz was able to deliver deep lesions (about 11 mm) through the intraventricular septum while sparing the adjacent right ventricular sub-endocardium (about 2 mm).56 This study did not have an RF ablation control group. Use of intravascular microbubble contrast agents during HIU failed to produce larger areas of necrosis.57
Stereotactic Body Radiation Therapy
Stereotactic body radiation therapy (SBRT) can deliver high-dose radiation to targeted tissue in a precise manner with minimal damage to adjacent tissue which may mitigate exposure to usual complications of invasive conventional VT ablation approaches.58 SBRT may have a role in the treatment of VAs as it can reach areas of inaccessible arrhythmogenic areas and achieve transmural lesions.
In a preclinical study, Lehmann et al. demonstrated the feasibility of SBRT in the atrial and ventricular myocardium of pigs.59 In five patients with treatment-refractory VT, Cucilich et al. showed that SBRT reduced the burden of VT after 12-month follow-up.60 Non-invasive electrocardiographic imaging (using a 256-electrode vest with a chest CT scan for anatomy) was used to aid in identifying the critical circuit for induced VT. A total dose of 25 Gy in a single fraction was administered to the target tissue while minimising the dose to surrounding tissue. On-table treatment times ranged from 11–18 minutes with no procedural complications or acute heart failure.
Preliminary results of the ENCORE-VT study (NCT02919618) demonstrated lower VA burden, fewer ICD therapies and improved quality of life in a cohort of 19 patients.61 At approximately 2 years, one patient developed a pericardial effusion, and another developed a gastro-pericardial fistula. According to early results of the STRA-MI-VT study (NCT04066517) seven patients received CT-guided SBRT with four patients surviving up to 6 months follow-up.62 At both 3 and 6 months, the number of VTs and ICD shocks decreased in comparison to pre-SBRT. No serious procedure-related adverse events were reported. In a retrospective study (n=8), the highest arrhythmic benefit in terms of VT burden and ICD interventions was in 2 weeks to 3 months after SBRT, perhaps due to transcription and eventual replacement of gap junctions over time after SBRT.63 The authors surmised SBRT should be considered as a bailout strategy or bridge therapy until heart transplantation in refractory cases.
In a study of 10 patients with repeated ICD therapies and refractory to standard treatment, SBRT demonstrated a reduction in VT duration and a trend for reduction in ICD shocks.64 In a 17-patient series, including five patients with electrical VT storm, SBRT reduced VT burden by 1–7 weeks post-treatment.65 In patients who underwent cardiac transplant after prior SBRT (12–250 days), histopathology of explanted hearts demonstrated subendocardial necrosis surrounded by a rim of fibrosis at the ablated areas.66 In another single-centre series of eight patients who underwent SBRT for refractory scar-mediated VT, ICD therapies decreased from a median of 69.5 to 13.3 post-SBRT (p=0.036) during a median 7.8 months follow-up.67 Proton beam therapy has also been examined as another non-invasive approach for the treatment of VAs. In a preclinical study, scanned proton beam radiation in 25 animals demonstrated achievement of cellular apoptosis and lesion formation assessed by MRI and histological analysis at 12 weeks.68 Infarction due to coronary artery damage was observed in three of 20 animals and 11 subjects developed mild-to-moderate global pericardial effusions within the 8–12-week period.
SBRT is a non-invasive alternative for the management of VA in patients who are not suited to conventional catheter ablation. Data from clinical SBRT trials on the long-term suppression of VT and procedural safety are forthcoming.62,69 Improvement in non-invasive diagnostic imaging and refined certainty of effective dosing protocols validated in larger studies using SBRT may widen its role in the future. Proton beam therapy is another intriguing modality but has only been tested in animal models, however, recruitment for clinical trials is underway (NCT04392193).
Focused Electric Field Ablation (Preclinical)
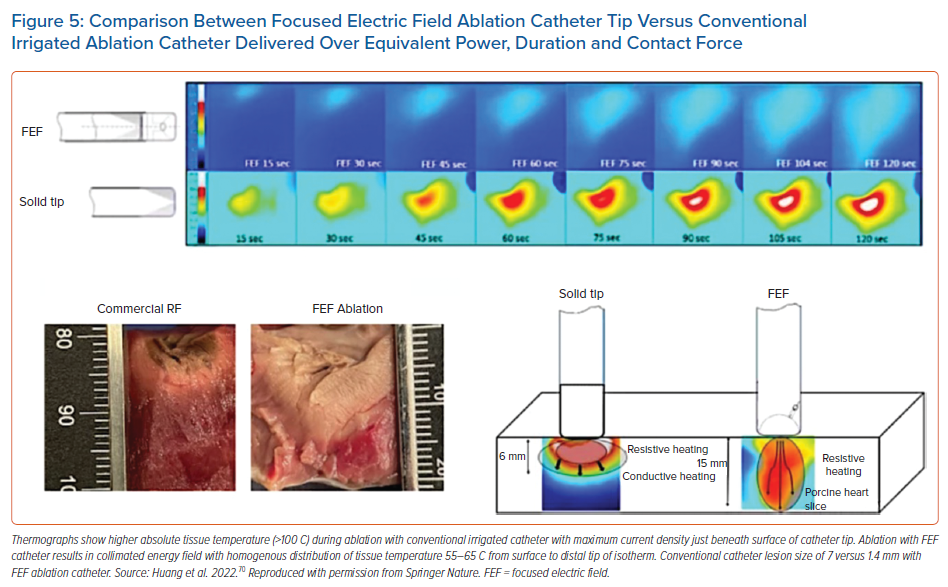
Focused electrical field (FEF) ablation is another novel technology under investigation. The FEF catheter uses a standard RF generator with minimal alteration of normal workflow for its use. As opposed to conventional RF catheters which achieve maximal current density – temperature – at the tissue interface, the FEF catheter uses a dome-shaped tip with a toroidal surface resulting in a collimated electric field and homogenous temperature distribution with more gradual fall-off at higher tissue depths. In an ex-vivo porcine model, Huang et al. demonstrated the FEF catheter created 12–14 mm lesions without SPs despite an RF duration of 2 minutes.70 Infrared thermal imaging showed maximal tissue temperatures 55–60oC in the FEF group (ablation time 120 seconds) versus maximum tissue temperatures of 90–100°C in the irrigated catheter arm (ablation time 90 seconds; Figure 5). Potential limitations of the current design are the requirement for perpendicular tip-tissue orientation and potential collateral thermal injury due to larger lesion size.71 Studies are being conducted to characterise the biophysics in scarred myocardium; investigate applicability for epicardial ablation; validate its effectiveness in a beating heart; and investigate its use as a PFA delivery source.
Alcohol Ablation Therapy
Alcohol ablation (AA) has been increasingly used as a bailout to ablation of intramural foci, especially with the increased use of epicardial and CVS activation mapping.72–74 This method may be performed with ethanol delivered through a transarterial or retrograde coronary venous approach.75–77 Limitations of the technique, particularly for transcoronary AA, are collateral injury to the conduction system and larger than intended infarction territory due to chemical reflux through collateral connections.78 Success of AA is also dependent on vascular anatomy and proximity of available vessels to the target region of interest and adequate control of occlusion during ethanol injection which may be reduced by the presence of collaterals.
Ultra-low Temperature Cryoablation
Ultra-low temperature cryoablation (ULTC) is a novel method of using a more powerful percutaneous cryo-energy system (with high-pressure nitrogen) than current commercially available cryoballoon/catheter technologies, which uses nitrous oxide). It has been shown to reliably produce contiguous, transmural and durable lesions over 3 months in ventricular myocardium of animal models with an average lesion depth of 5.6 mm.79 The ULTC system developed by Adagio Medical uses catheter shaped by endoluminal stylets to achieve circular, linear and focal lesions. In a recent study, ULTC was found to be safe and effective in 79 patients with AF undergoing pulmonary vein isolation and posterior wall isolation.80 In a preclinical study, combining ULTC and PFA was found to be safe and effective in the atrium.81 A single-arm, multicentre, open-label study is underway to evaluate ULTC in patients with monomorphic VT (CryoCureVT, NCT04893317). This could be a promising modality for VT ablation in the future, particularly if cryothermal-induced local tissue impedance changes positively affect subsequent electrical field PFA delivery using the combined ULTC and PFA approach.
Conclusion
Novel technologies under investigation certainly show promise for increasing lesion size in a safe manner, but it remains to be seen if such techniques will offer superior alternatives to RF ablation in clinical practice. Head-to-head clinical studies will be critical in defining their future role for VT ablation. 
Clinical Perspectives
- Radiofrequency ablation has become the cornerstone modality of catheter ablation for treatment of ventricular arrhythmias but limitations remain when putative isthmuses lie within deep substrate.
- Modified workflows using conventional radiofrequency catheters may improve lesion size but may have greater risk of complications.
- Integration of cardiac imaging with 3D anatomical mapping and entrainment may help identify concealed areas of mid-myocardial substrate for lesion targeting.
- New catheter-based or non-invasive lesion delivery technologies may provide additional effective methods for targeting ventricular arrhythmias for challenging substrates.