The implantation of permanent transvenous pacemakers has long been established as the first line treatment for patients with bradyarrhythmias. Continuous device improvements and an ageing population have led to a corresponding increase in implantations, with approximately 1,000 units per million people implanted annually in Europe.1
However, transvenous pacing still has several limitations, leading to significant complications in 9–12% of patients.2,3 Complications may be acute (<30 days after implantation) and can include bleeding/haematoma, pneumothorax, pericardial effusion/perforation, infection and lead displacement. Chronic complications include lead fractures and infections, with rates particularly high at the time of generator change.
The development of leadless pacemakers was intended to address some of the limitations seen with transvenous pacemakers. The first leadless pacemaker was implanted in 2012. In all, 1,423 Nanostim devices (Nanostim Inc./St Jude Medical/Abbott Medical) were implanted before the device was withdrawn due to several cases of premature battery depletion.4
The first Micra transcatheter pacing system was implanted in 2013 (Micra transcatheter pacing system; Medtronic) and, to date, almost 150,000 devices have been implanted worldwide. The safety and efficacy of this device have been studied extensively. During trials, the utility of this device was demonstrated, with a 99% successful implantation rate (719 patients of 725 recruited) and a 96% primary safety end point (patients should be free of system- or procedure-related major complications).5 Registry data following the investigational device exemption study continue to demonstrate 99% procedural success rates and low complication rates (2.7% at 12 months).6 The second-generation Micra transcatheter pacing system uses the signal generated by the device’s accelerometer to sense atrial activity and then sequentially pace the right ventricle, providing a VDD pacing mode. Initial studies demonstrated a mean atrioventricular rate of synchrony of 87%.7
A key advantage of using leadless pacemakers over transvenous devices is the marked reduction in pacemaker-related infection. Pacemaker-related infections occur in 7–12% of cases of transvenous pacemakers, and the risk triples in replacement procedures.3,8 During clinical trials of leadless pacemakers, there was an absence of pacemaker-related infections, even in bacteraemia settings.3,2 It is likely that this is the result of encapsulation of the device within the right ventricle and the absence of leads in the vasculature and generator on the chest wall.
Although there is currently no head-to-head randomised controlled trial for leadless devices again transvenous pacemakers, the currently available evidence base suggests that leadless pacemakers have favourable complication rates, with a 63% lower rate of complications than transvenous devices.6 As the number of devices implanted increases, the literature identifies certain patient populations where leadless pacing is considered advantageous. This includes patients with prior cardiac device infection, patients on haemodialysis and patients in whom there is an expectation of low levels of pacing in a young population (e.g. cardioinhibitory vasovagal syncope).8–11
Despite these advantages, current guidance within the UK limits the use of leadless devices only for the purposes of research or when conventional pacemakers are contraindicated.12 Although the 2021 guidelines from the European Society of Cardiology (ESC) state that leadless devices can be used when the risk of infection is high, incorporating shared decision- making and taking into account life expectancy considerations, leadless pacing remains a relatively niche procedure.13
Recent efforts have been made by groups of Austrian and Polish healthcare professionals (HCPs) to identify the indications and contraindications for the wider use of leadless pacemakers, developing a set of criteria through which this could be achieved within their healthcare settings.3,14 Given the state of leadless pacemaker implantation and the positions taken by the Austrian and Polish researchers, the intent of this study was to determine how leadless pacing could be more optimally used within the UK NHS.
A comprehensive literature review on leadless pacemakers was compiled and presented to a panel of experts in leadless pacing device implantation from across the UK. The panel convened in January 2022 to discuss current challenges around the optimal clinical use of leadless pacing. Using a modified Delphi methodology guided by an independent facilitator, the panellists identified five main topics of focus:
- problems that are experienced with transvenous pacing and need to be appreciated/acknowledged;
- the relative risk of leadless systems;
- patient types suitable for leadless pacemakers who may be at risk from transvenous devices;
- the role of a national register; and
- logistical requirements for the safe delivery of leadless pacemakers in the UK.
These topics were discussed further, with 36 statements developed and used to create an online questionnaire using Microsoft Forms. The questionnaire was distributed to 72 leadless implanters identified as working within the UK by PRR. Stopping criteria were agreed as a 3-month time period to collect responses (February–April 2022), a minimum 25% response rate, and at least 75% of statements achieving the agreement threshold for consensus. These criteria were set to allow for the greatest number of HCPs to respond given the pressures currently being experienced by the health service in relation to the COVID-19 pandemic. Given the speciality of the field, the threshold for consensus agreement was set at 66%. Consensus agreement was further defined as ‘high’ at ≥66% and ‘very high’ at ≥90%.
Respondents used a 4-point Likert scale (strongly disagree, tend to disagree, tend to agree and strongly agree) to indicate their corresponding level of agreement with each statement. The questionnaire also captured some demographic data for further analysis, including years of experience in implanting cardiac pacing devices, years of experience in implanting leadless devices and the number of leadless devices implanted per year.
Completed anonymised surveys were collated and analysed by an independent facilitator to produce an arithmetic agreement score for each statement. This information was then reviewed by the panel of experts to determine what recommendations could be made based on the responses received.
Because this study only sought the anonymous opinions of healthcare professionals, ethics approval was not sought. However, a statement of consent was provided at the start of the survey, and all completing participants provided consent in line with this statement.
Outcome of the Delphi Process
Of the 72 implanters identified, four could not be contacted for inclusion in the study; thus, 68 invitations sent out. Of these, 27 responses were received (40% response rate) and analysed.
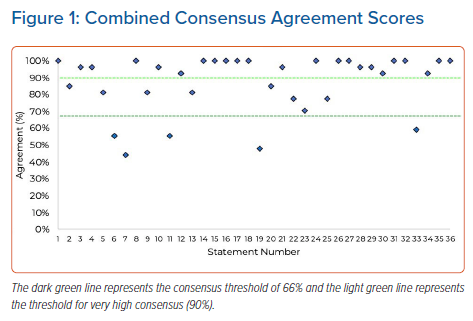
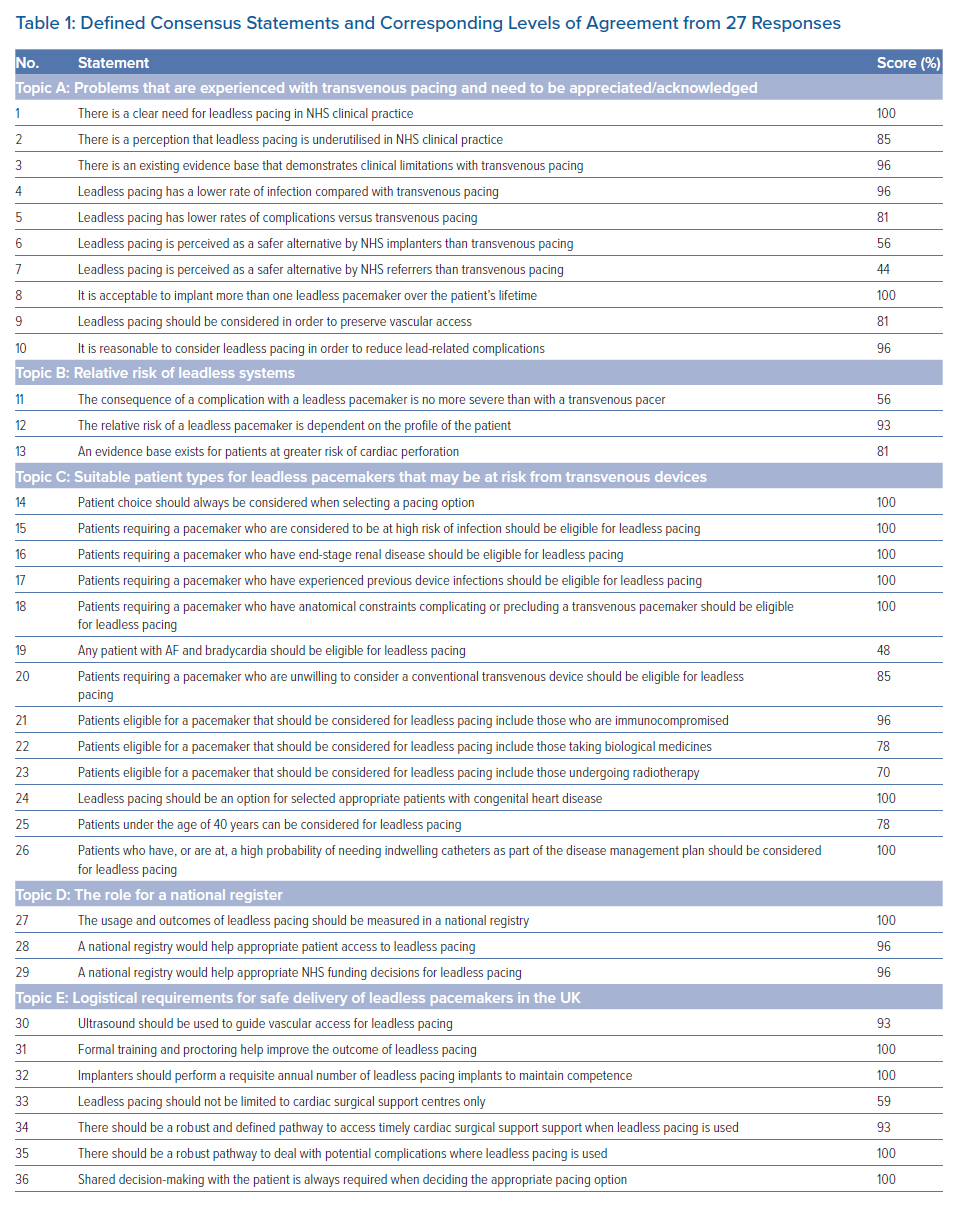
From the first round of consensus, 23 of 36 statements attained very high (≥90%) agreement, eight attained high (<90% and ≥66%) agreement and five did not reach the threshold for consensus (<66%; Figure 1; Table 1). Given the high level of agreement attained for the statements and that the stopping criteria had been met, it was decided not to undertake a second round of testing.
The results demonstrate a strong degree of support for most statements, with more experienced clinicians showing a lower degree of support overall than more junior colleagues (Supplementary Figure 1). However, this association was less clear when examining the experience of respondents with implanting leadless devices (Supplementary Figure 2).
Discussion
Perception of the Safety of Leadless Pacemakers
It is clear from the level of agreement with Statements 6 and 7 (Table 1; 56% and 44%, respectively) that respondents are unclear as to the perceptions of the wider healthcare community around the safety of leadless pacemakers.
During discussion of the results, the panellists agreed that it is a challenge to know what other HCPs, especially those who refer patients on for pacemaker implantation, think about the safety and use of a leadless device over a more traditional transvenous pacemaker. It was also noted that, to date, patients offered leadless devices are those who are at greater risk of a complication to begin with, which therefore may inversely affect the perception of the safety of the device.
The panellists suggested that this is an area where improvements could be made by expanding the education around leadless pacemakers so that clinicians and referring colleagues are more aware of the advantages of the systems and how they can be used to improve patient outcomes.
Which Patients Benefit Most From a Leadless Pacemaker
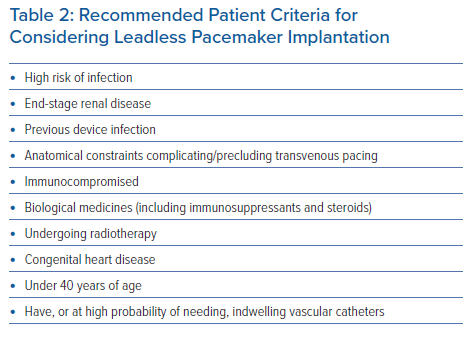
Part of the intent of this study was to define suitable patient types who would benefit from leadless pacemaker implantation. This would build on the findings of previous studies to help establish the position of UK implanters. Based on the agreement from Statements 15–19 and 21–26 (Table 1), the panellists offered patient criteria for considering leadless pacemaker implantation, as presented in Table 2.
It is possible that the sub-threshold agreement level for Statement 19 (48%) indicates that the responders considered that single-chamber transvenous pacemakers were entirely reasonable in an uncomplicated population with AF and bradycardia. AF with bradycardia is supported as a basic criterion for leadless pacemaker implantation in both the ESC 2021 guidelines and within the study examining the position of Austrian HCPs conducted by Steinwender et al.3,13
Most of the recommended patient populations relate specifically to complications associated with transvenous systems that are mitigated by a leadless pacemaker. Infection has been recognised as a very remote complication of leadless pacemakers, with no devices having to be removed as a consequence of infection in either the investigational device exemption study or the postapproval registry.1,6 Consequently, this device is attractive for patients who are at a high risk of infection, including those on haemodialysis, those with a previous cardiac device infection, those who are immunocompromised, those undergoing steroid therapy or receiving biological drugs and those with indwelling vascular catheters. Other recommendations are largely justified by the anatomical advantage of not having leads in blood vessels or a generator (i.e. patients undergoing thoracic radiotherapy, younger patients and patients with congenital heart disease who may be younger and not have appropriate venous access for transvenous pacing).
Further to this list, the cost of the device should be taken into consideration because there is variation across the UK. Therefore, the panellists recommended that leadless devices should be used in a targeted approach that takes into account patient experience and quality of life factors.
National Register Needs
The strength of the response to Statements 27–29 suggests that implanters recognise the need for a specific register to capture information around the use of leadless devices, including their risks and complication rates. The panellists suggested that these data should be input by implanters to ensure accuracy. Furthermore, the panellists agreed that the National Institute for Cardiovascular Outcomes Research (NICOR) database is not currently able to manage the information needs of leadless pacemakers, but that it could be expanded to provide the appropriate fields. However, it is beyond the scope of the present study to provide recommendations as to how this should be achieved.
Logistical Requirements for Delivering Leadless Systems
There was consensus that ultrasound should be used when implanting leadless pacemakers. It has been demonstrated that complication rates for femoral access for electrophysiology procedures are lower if ultrasound is used.15 In that meta-analysis of 7,858 patients, the incidence of vascular complications in the ultrasound group was 1.2%, compared with 3.2% in the anatomic landmark guided group (p<0.00001).15 Because the introducer sheaths for leadless devices are large (e.g. 23 Fr), it would seem logical that safety would be enhanced if ultrasound was used. The low complication rate and high success rates associated with leadless pacemaker implantation may be attributed, in part, to the extensive training available for this procedure and the experience of operators. Consequently, maintaining this high level of training and ensuring ongoing experience with recommended minimal annual numbers would seem appropriate, and was reflected by consensus on these points.
The evidence base on the Micra device indicates that the incidence of pericardial perforation requiring surgical intervention is low. In the postapproval registry, two of the 1,817 patients recruited (0.1%) required surgical intervention6. Despite this low number, there was no consensus about undertaking leadless pacing in non-cardiac surgical centres. However, there was consensus that centres should have a defined pathway in place to access cardiac surgical support. This would include procedures performed in a cardiac surgical centre and a non-cardiac surgical centre. In the latter situation, the process would be similar to that for the rare occasions when percutaneous coronary intervention or AF ablation require surgical input. This would need to be a predefined process of urgent transfer, recognising that any delay may adversely affect outcome. Similarly, it was recognised that centres implanting leadless pacemakers should have robust pathways in place to address any complications associated with the device or the procedure.
The use of shared decision-making is widely acknowledged as an important part of patient care and features highly within the NHS Long Term Plan, as well as General Medical Council guidance on consent.16,17 Not surprisingly, the use of shared decision-making in deciding on leadless pacing reached 100% consensus.
Recommendations
Based on the levels of agreement from 27 responses, the authors offer the following set of recommendations:
- Education for implanters and referrers regarding the benefits and safety of leadless pacing systems should be improved.
- Awareness and training on the use of leadless devices should be improved for non-leadless implanters.
- A registry should be developed to track the complications and risks associated with the use of leadless devices.
- Leadless devices should be more widely used so that implanters can better understand and mitigate the risks involved with the device.
- Leadless pacemakers should be considered in certain patient populations (Table 2).
- The choice to use a leadless pacemaker should be clinically driven to ensure the best outcome for the patient.
- A robust and defined pathway for timely cardiac surgical support for leadless pacing should be developed.
The results of this study are a representative sample of the opinions of implanters currently operating within the field. This provides a useful basis for the panel to propose recommendations to improve the use of leadless devices on a patient-centred basis.
As with all consensus studies, the wording of statements may have affected the levels of agreement attained. Future work could refine the statements found less agreeable in the present study to determine what elements are driving the agreement shown.
Conclusion
This consensus document is based on the expert opinion of 27 leadless pacemaker implanters currently operating within the UK, representing a response rate of 40%. The results provide a strong indication of the opinions of these specialists.
This study highlights that there are elements within the current approach to the use of leadless pacemakers that should be modified to improve the clinical utility of the device with a patient-centric focus, including patient types suitable for implantation, the role of a national register and the logistical requirements for delivering the system.
The implementation of the seven recommendations listed above may increase the use of leadless pacemakers, with the aim of improving patient outcomes.
Click here to view Supplementary Material.
Clinical Perspective
- Leadless pacing appears to be a safe and effective alternative to conventional transvenous pacing.
- A Delphi model was used to evaluate opinions on aspects of leadless pacing in the UK, including problems associated with transvenous pacing, risks of leadless pacing, patient types for leadless pacing, the role of a national register and the logistics of delivering leadless pacing.
- The results of the Delphi process and expert opinion resulted in seven recommendations, including the need for a national register.