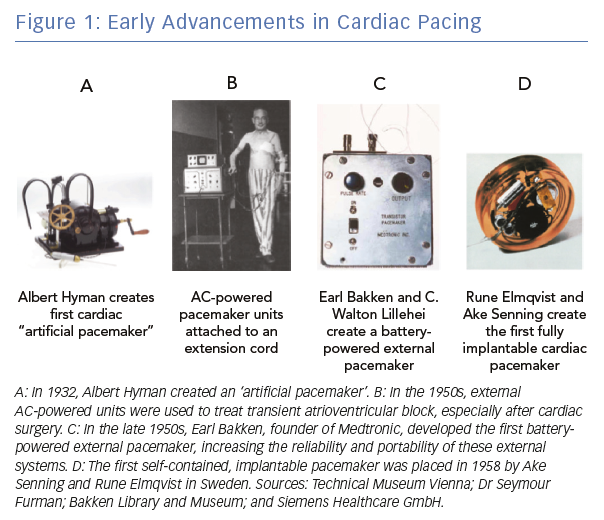
A Brief History of Cardiac Pacing
Electrical stimulation of the heart was used sporadically throughout the 19th century, generating a set of case reports, largely related to attempts to resuscitate people.1 The contemporary field of cardiac pacing emerged in the 20th century (Figure 1). The first use of pacemakers in a modern sense was in the late 1920s when Australian anaesthetist Mark Lidwell and American physiologist Albert Hyman, working independently of each other, developed the first cardiac pacemaker machines. Dr Hyman created the first device to artificially pace the heart in 1932 and coined the term ‘artificial pacemaker’.2 At this time, however, the invention was regarded with scepticism and was not widely adopted.
In the 1950s, significant breakthroughs in the field of cardiac pacing occurred. During this time, Paul Zoll created a completely external system for transcutaneous pacing, a system that is still in use today in emergencies.3 While effective, this technique was limited due to the use of high voltages and the resultant painful stimulation from external thoracic pacing. At this time, the field of cardiac surgery was rapidly advancing and a frequently encountered complication was damage to the His bundle with resultant atrioventricular (AV) block. Electrodes directly attached to the heart could be connected to an external generator to stimulate the heart and allow for recovery of the conduction system. C Walton Lillehei and Earl Bakken, from the University of Minnesota, developed a battery-powered external pacemaker, obviating the need for an external power source and making the system more reliable and portable.4
In 1958, Swedish physicians Ake Senning and Rune Elmqvist implanted the first fully internal pacemaker.4 The pulse generator was placed in the epigastrium and was attached to epicardial leads. The first patient to receive this device, Arne Larsson, underwent 26 pacemaker procedures over the course of his lifetime and died at the age of 86, outliving his original physicians.5 Subsequent improvements in the battery technology, the size and programmability of the pulse generators, and the use of transvenous components have led to our modern pacemaker systems. However, the overall concept and design has not changed significantly since the mid-1960s.
Limitations of Current Pacemaker Systems
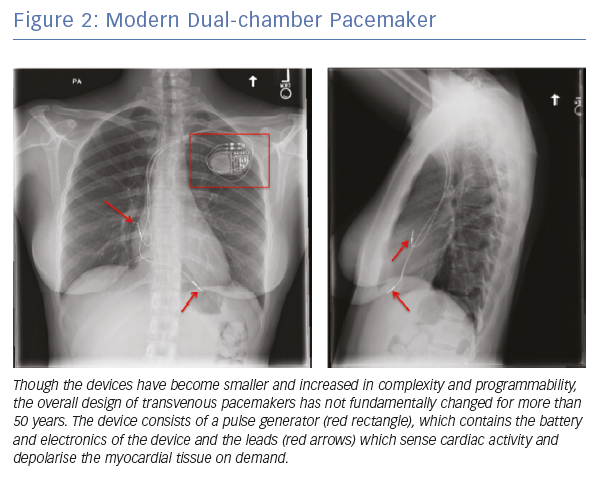
Current transvenous pacemakers consist of a pulse generator, which contains the battery and electronics, and the leads that travel from the pulse generator and contact the myocardium to sense cardiac activity and deliver electrical impulses (Figure 2). These systems are effective for the treatment of symptomatic bradycardia; however, the overall design has not significantly changed in more than 50 years.
Many of the limitations and complications of these devices are related to their overall design and construction, particularly the leads. The leads consist of an insulation-encapsulated conductor that is repeatedly subjected to cardiac and shoulder motion, which may result in mechanical stresses that can cause them to fracture over time. The pulse generator sits in an extravascular space and can serve as a nidus for bacterial infection with the leads serving as a portal of entry into the bloodstream. In addition, the fundamental approach to transvenous pacing, that the native His-Purkinje system can be bypassed and replaced with non-physiological electrical stimulation, has been shown to result in reduced cardiac synchrony and efficiency with worse clinical outcomes in some situations.
Implant-related Complications
Complications from pacemaker implantation can be divided into immediate, intermediate and late-term complications. The rates of complications range from <1% to 6% and require prompt recognition and management.6
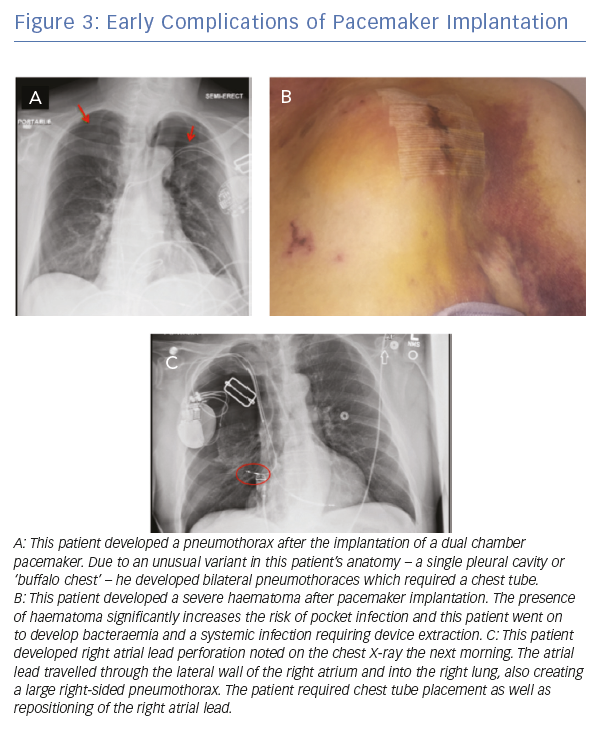
Immediate, procedure-related complications are related to the implant process and can include pneumothorax and haemothorax, pocket haematoma, cardiac perforation and lead dislodgements (Figure 3).
Intermediate complications include device infection, venous thrombosis or stenosis, pain or discomfort at the pocket site, mechanical disruption of the tricuspid valve with resultant tricuspid regurgitation and, rarely, discomfort with ventricular pacing.
Late complications can include lead fracture or insulation breaks due to mechanical stresses, increases in pacing threshold or impedances due to tissue ingrowth, and device infections, often with systemic bloodstream infection. Treatment of the late-term complications of device implantation is often complicated and may require lead extraction, which can be technically challenging and carries a risk of central venous or cardiac perforation, haemothorax and death.
Recent Advancements in Cardiac Pacing
The basic design of cardiac pacing devices has not significantly changed since the mid-1960s and their limitations are well known. Recent advancements have attempted to address these limitations by reducing hardware and improving cardiac efficiency. These attempts include the advent of leadless pacemaker systems as well as attempts to improve cardiac efficiency with permanent His bundle pacing (PHBP), algorithms designed to mimic normal physiology and new technologies for cardiac resynchronisation therapy (CRT).
Leadless Pacemakers
Leadless pacemakers represent a fundamental paradigm shift in the design of pacemaker systems with the goal of creating small, completely intracardiac units without transvenous leads and disconnected from any extravascular components. Two designs have been explored for leadless pacemakers – single and multicomponent systems.
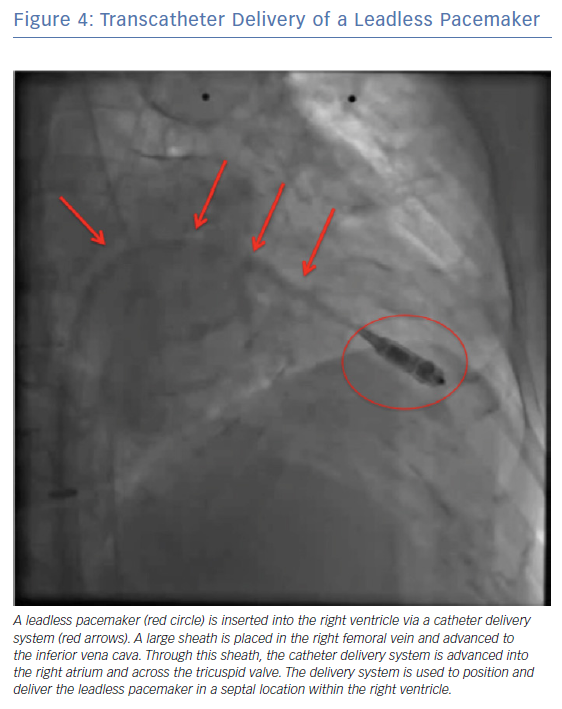
A single-component system has an individual, small unit which contains the entire pacemaker (battery, electronics and electrodes) which is implanted in the heart using a deflectable delivery sheath (Figure 4). This simple design allows easy implantation of an energy-efficient system and eliminates the need for extravascular components and leads.
The commercially available leadless pacemakers that are currently used are of this design. However, there are limitations of this system, most notably the difficulty with device retrieval for infection, premature device failure, or battery depletion. There are also uncertain risks such as thrombus formation and risk of infection. In addition, the currently available systems can only be used for single chamber ventricular pacing (VVI or VVIR), limiting their widespread applicability. For most patients with sinus node dysfunction and AV block, a single chamber leadless pacemakers is a suboptimal choice compared with a dual chamber pacemaker; therefore, they are largely limited to patients with permanent AF and slow ventricular response or those with paroxysmal, infrequent AV block.7 Efforts are currently underway to develop a dual chamber design, though challenges with device-to-device communication and active fixation in the thin-walled right atrium will need to be overcome.
Two leadless pacing devices were available in the US, including the Nanostim™ leadless cardiac pacemaker (Abbott) and the Micra™ transcatheter pacing system (Medtronic). However, the Nanostim device was recalled in 2016 due to issues of premature battery depletion and is not currently available. In clinical trials, both systems demonstrated a high rate of successful implantation (>95%).8,9 However, there was a 4–6.5% rate of major complications, including perforations or pericardial effusions in 1.5–1.6% of cases.8,9 Pacemaker measurements were stable at 6 months.8,9
In analyses comparing patients with leadless pacemakers to a cohort of patients with transvenous pacemakers, there were fewer short and intermediate-term complications with leadless pacing, largely driven by an absence of lead and pocket complications and a low risk of infection.9,10 Dual chamber leadless pacing systems are under development. There are also efforts to provide AV synchronous pacing using a ventricular leadless pacemaker that senses atrial contraction to provide VDD pacing.
Multicomponent pacemaker systems are of a different design and are not commercially available. Rather than relying on a single unit containing all electrical components of the device, a smaller endocardial ‘seed’ is used and functions as an energy transducer. A second, extrathoracic device communicates with the endocardial component using energy to induce a pacing pulse. This design could allow for dual-chamber pacing as well as cardiac resynchronisation therapy. In addition, a system that integrates a subcutaneous ICD (S-ICD) as the extrathoracic component would allow for bradycardia pacing and anti-tachycardia pacing.
Permanent His Bundle Pacing
Chronic RV apical pacing is non-physiological and has been associated with an increased risk of heart failure, AF and death. This has prompted a search for alternative sites for pacing including the high septum, right ventricular outflow tract (RVOT), moderator band and His bundle.Capture of the His bundle allows rapid, efficient activation of the ventricles by using the Purkinje network. While the concept of His bundle pacing has been around since the 1960s, there was limited clinical experience with it as a pacing technique until a small study by Pramod Deshmukh in 2000.11,12 At that time, there were no specific tools to help accomplish the task and PHBP has only recently become widespread.
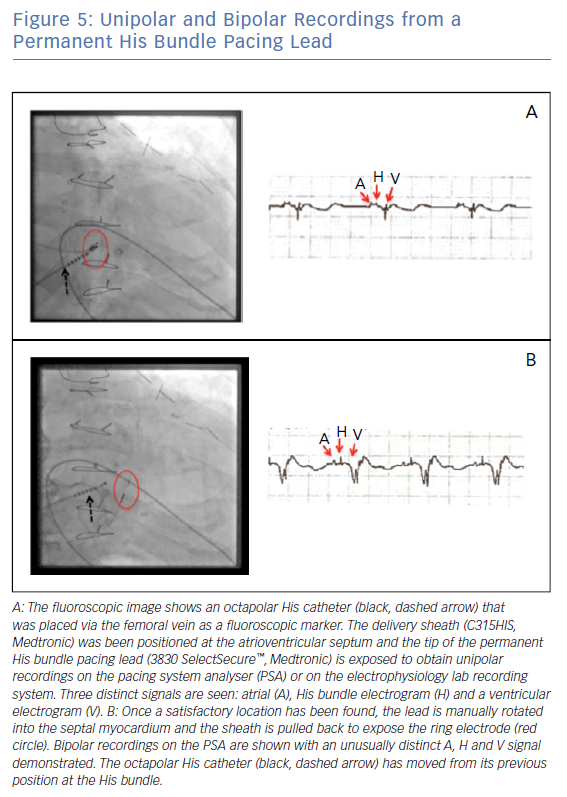
A small-calibre, lumenless pacing lead (Medtronic’s 3830 SelectSecure™) is delivered via a specially designed sheath (C315HIS, Medtronic) to map the AV septum. Unipolar recording and pacing can be used to locate a His bundle electrogram near the membranous septum (Figure 5). When an appropriate location is found, the lead is manually rotated to fix it to the myocardium in a location where the cardiac conduction system can be captured. If His bundle capture is confirmed at this location and pacing thresholds are acceptable, the delivery sheath is slit and final capture thresholds are measured.
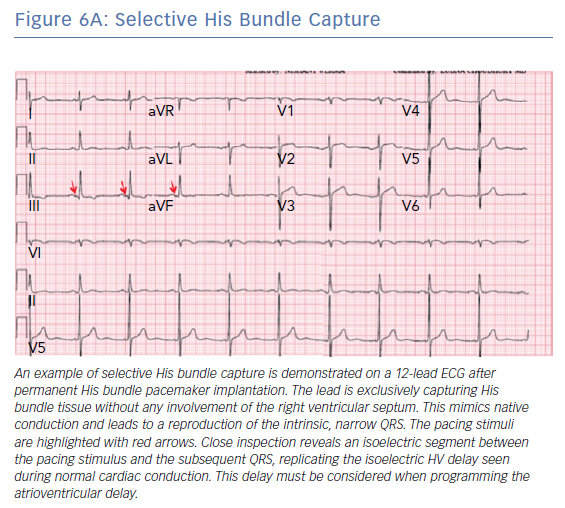
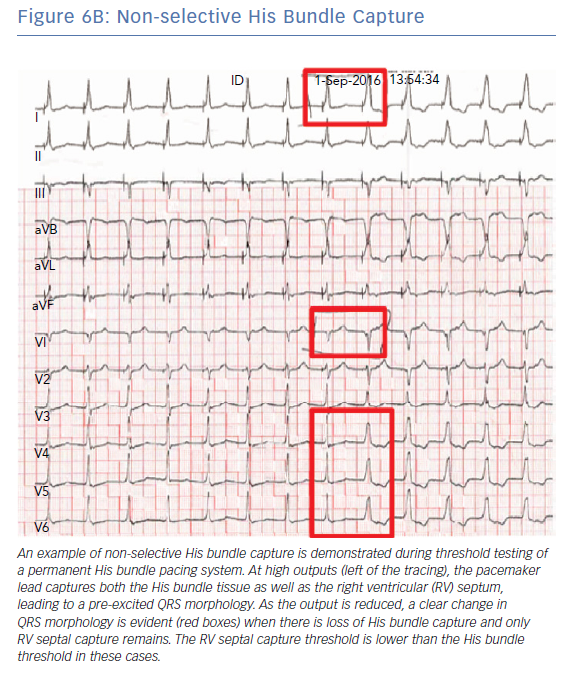
A number of different QRS morphologies can be seen with His bundle pacing because the tissues that can be captured in this area include the His bundle, RV septum and right atrium. Standardised nomenclature have been developed to describe the various electrocardiographic findings including selective His bundle capture (His bundle capture alone; Figure 6A) and non-selective His bundle capture (His bundle capture along with RV septal capture; Figure 6B).
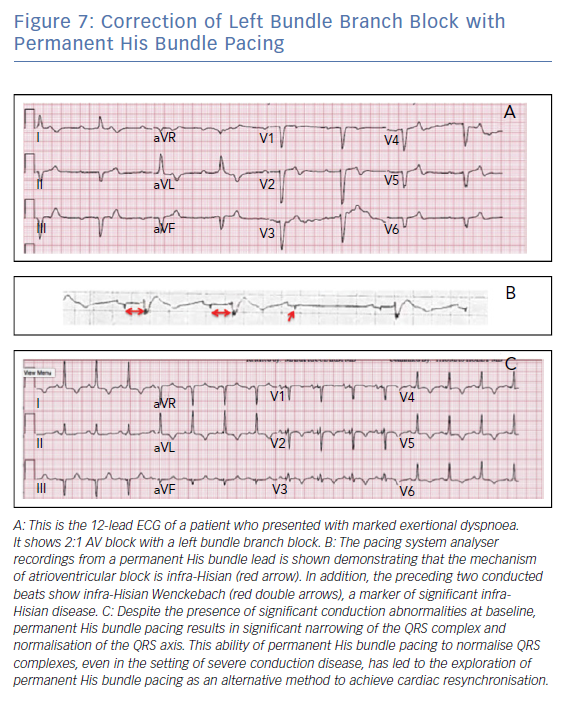
Data have shown that the implant success rate for PHBP ranges from 70–90%.13,14 Lead thresholds tend to be higher than traditional RV apical leads; however, the thresholds appear to remain stable over time. PHBP was found to have a statistically significant reduction in a combined endpoint of hospitalisation for heart failure, death or upgrade to biventricular pacing when compared with patients undergoing traditional RV apical pacing (HR 0.71; p<0.02). This effect was most pronounced when the RV pacing burden is more than 20% and was largely driven by a reduction in heart failure events.13 This technique has also been shown to be effective in instances of complete AV block and in patients with right and left bundle branch block (Figure 7). This reversal of conduction disease is generally explained by the concept of longitudinal dissociation in the His bundle. However, other explanations include differential source-sink relationships during pacing versus intrinsic impulse propagation, virtual electrode polarisation and local capture of conduction tissue fibres that connect downstream from the site of bundle branch block.15,16
The ability to reverse left bundle branch block (LBBB) and normalise the QRS has led to an interest in using PHBP for CRT.17 Additional data will be needed from upcoming trials to compare these two methods of resynchronisation. The recently published His Bundle Pacing Versus Coronary Sinus Pacing for Cardiac Resynchronization Therapy (His-SYNC) trial was the first multi-centre, prospective trial comparing PHBP with coronary sinus (CS) lead implantation for CRT candidates (NCT02700425).18 There was no difference in outcomes seen between the PHBP group and traditional CS group in this pilot study; however, there was a high crossover rate between the two groups. A method involving transseptal, direct left bundle stimulation from the RV has achieved QRS normalisation, even in patients where the LBBB cannot be corrected with PHBP.19
Closed Loop Stimulation
In an effort to improve upon the traditional rate-drop response algorithm and create a more physiologic response to the need for pacing, closed loop stimulation has been developed by Biotronik. The algorithm measures RV impedance, a surrogate for cardiac contractility, and uses this information to adjust the pacing rate before a sudden drop in heart rate. There has been some success with the treatment of vasovagal syncope with this algorithm although a large, multicentre, randomised clinical trial, Benefit of Dual Chamber Pacing with Closed Loop Stimulation (CLS) in Tilt-induced Cardioinhibitory Reflex Syncope (BIOSync CLS) study (NCT02324920), is still ongoing.20
Cardiac Resynchronisation Therapy
Ventricular cardiac dyssynchrony arises when segments of the left ventricle (LV) contract late due to delayed electrical activation. Most commonly, this occurs in the lateral wall of the LV due to LBBB. CRT uses the concept of targeted electrical stimulation to treat a delayed segment of the ventricle in order to improve electrical and mechanical synchrony and efficiency. This is accomplished through near simultaneous activation of an RV apical lead and an epicardial LV lead placed via the CS. CRT is not always effective and up to 30% of patients do not respond to this therapy. However, efforts to maximise the response from CRT include appropriate patient selection as outlined by existing clinical trial data, CS lead placement in an optimal location for resynchronisation and delivery of CRT therapy with every cardiac cycle.
Multisite and Multipoint Pacing
A significant recent development in the field of CRT was the creation of the quadripolar CS lead. Before this, unipolar and bipolar leads for CRT were hampered by limited programmability, resulting in high pacing thresholds or phrenic nerve capture with even minor changes in lead position. Quadripolar leads were more forgiving, offering multiple options for pacing vectors allowing for more targeted cardiac resynchronisation. These leads were found have a lower risk of lead replacement and abandonment and appear to improve mortality when compared with bipolar leads.21 A natural extension of these benefits is the concept that pacing from more than one LV site may allow for improved resynchronisation and outcomes.
Multisite pacing refers to the concept of using two or more epicardial CS leads to improve the resynchronisation response. Implantation of two or more CS leads has been shown to be feasible and safe. Small studies have shown benefits of this technique in haemodynamic response, ejection fraction, LV end systolic volume and heart failure symptoms.22 However, the use of a Y-adaptor to pace between the two leads can result in the use of high outputs and early battery depletion.23 Additional studies are needed for effectiveness of this technique as the results have been mixed.24
Multipoint pacing refers to pacing from multiple LV sites through a single quadripolar lead. By choosing widely spaced electrodes, a large region of LV myocardium can be captured to provide the best haemodynamic response. This option appears safe and feasible and small studies have shown haemodynamic advantages to multipoint pacing.25 However, a recently published study has shown no initial benefit with multipoint pacing in patients who are CRT non-responders.26 Additional phase II data from this study and other ongoing studies will be needed to assess whether there is a significant advantage to this technique.
Endocardial LV pacing
Epicardial CS lead placement, while successful, is hampered by multiple mechanisms of non-response. Fundamentally, it is a non-physiologic form of pacing as cardiac electrical activation normally proceeds from the endocardium to the epicardium. In addition, CS lead placement relies on the existing coronary venous anatomy, which may be unsuitable for optimal lead placement.
Endocardial LV lead placement has been explored as an alternative to allow for more targeted LV lead placement with a more physiologic ventricular activation and no risk of phrenic nerve capture. This has been accomplished by using conventional pacing leads placed through the interatrial or interventricular septum, as well as a leadless system that involves retrograde aortic implantation of a wireless, endocardial pacing electrode.27–29 Despite the attractive features of this technique, even those studies that have shown some indication of clinical benefit have shown a high rate of adverse events, including systemic thromboembolism and procedural complications.29,30 Additional improvements in the delivery systems, increased familiarity with anticoagulation requirements for left-sided lead implantation and further studies will hopefully help to reduce complication rates and improve outcomes with this technique.
Future Directions
Future developments for pacemakers will see a continued reduction in hardware. In current pacemaker systems, battery depletion and subsequent generator changes present additional risk of complications, especially infection. Preliminary work has been done to look at using flexible sheets of piezoelectric wires to convert cardiac motion to energy to power pacemaker devices in a nearly inexhaustible manner.31 There have also been efforts at creating biologic pacemakers using gene therapy to increase automaticity of existing non-pacemaker cardiac myocytes.32,33 This research is still in its early stages.
Conclusion
Pacemakers were initially developed in an effort to prevent catastrophic, bradycardic events. These simple devices have evolved into modern systems with significant complexity and a high degree of programmability. However, the overall design of pacemaker systems has not significantly changed in more than 50 years, until recently.
The advent of leadless pacemaker systems and the adoption of permanent His bundle pacing represent paradigm shifts in the field of cardiac pacing, and have brought a renewed sense of excitement and interest to the field of bradycardia pacing. These efforts and future innovations are likely to continue to focus on advancements that allow for further reductions in hardware and improvements in cardiac efficiency with a goal to reduce complications and improve clinical outcomes.
Clinical Perspective
- Cardiac pacing design had remained largely unchanged for more than 50 years.
- Leadless pacemakers represent a paradigm shift in the design of pacemaker systems, allowing for a significant reduction in hardware and potential reduction in complications.
- Permanent His bundle pacing represents a fundamental shift in the approach to cardiac pacing, focusing on physiological stimulation of the cardiac muscle to improve cardiac efficiency.
- Multisite and multipoint pacing represent efforts in the field of cardiac resynchronisation therapy to improve cardiac efficiency and improve clinical outcomes.