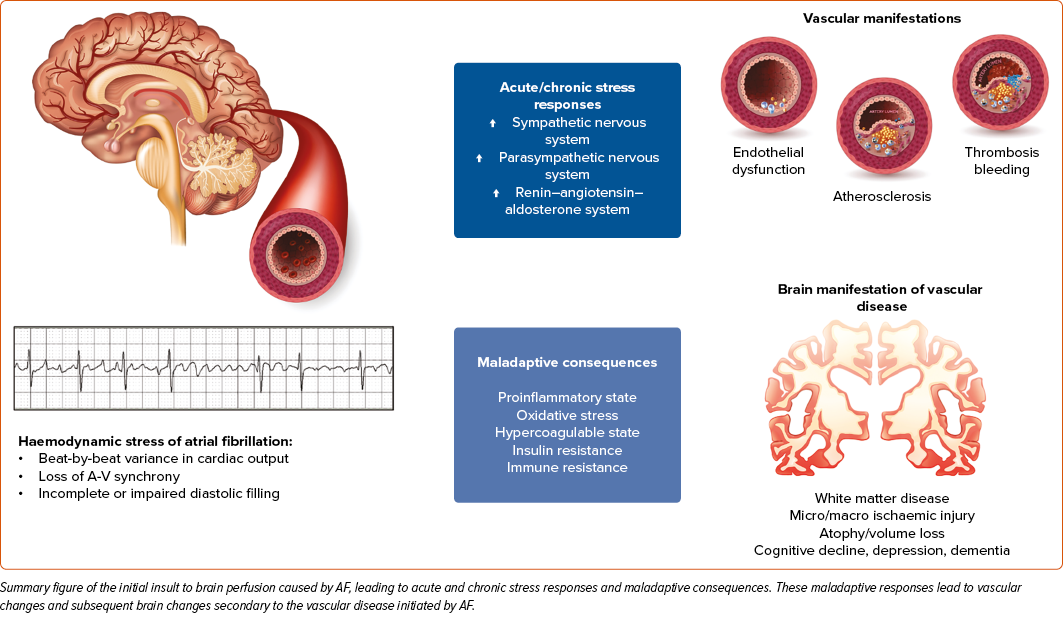
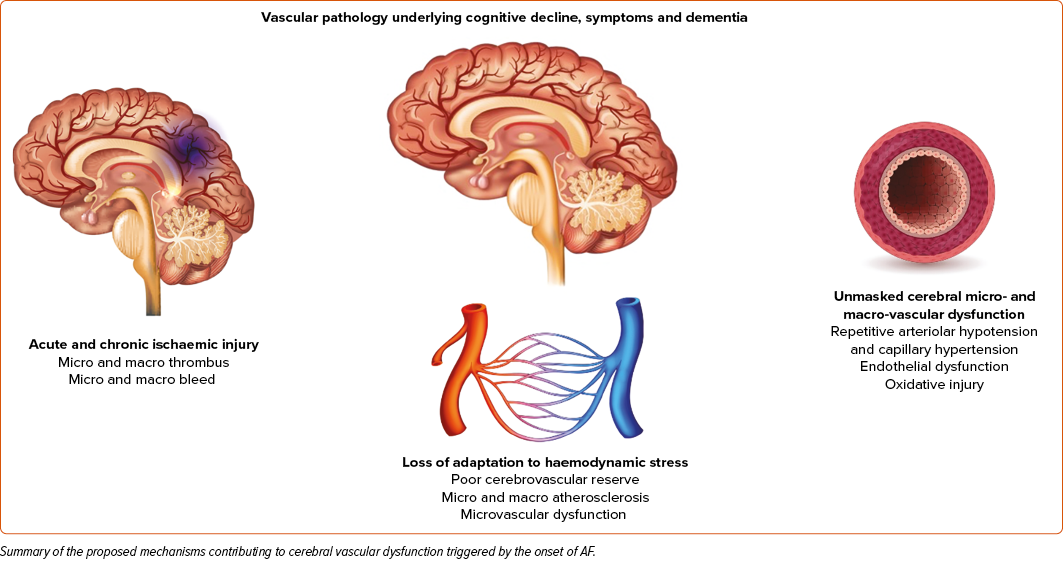
AF is an independent predictor of cognitive decline and dementia.1–4 Specifically, AF has been shown to increase the risk of dementia progression in patients and has been correlated with decreased cognitive assessment scores over time.1,2,5 A recent meta-analysis of 18 studies that included 3,559,349 patients identified an increased risk of cognitive decline in those with AF of approximately 40–60%.6 The reasons for this cognitive decline are not clearly understood, but are likely multifactorial.1,2,5,7 Several hypotheses have been proposed with varying levels of experimental evidence, and include macro- or microvascular stroke events, biochemical changes to the blood–brain barrier related to anticoagulation, or hypo- or hyperperfusion events.1,2,5
Brain health and progressive cognitive dysfunction related to clinical and subclinical strokes and vascular dementia have been well described.2,4,5,7,8 Consequently, the most extensive studies have focused on the role of thromboembolic events that occur during AF and the ensuing cognitive decline.2,5 However, even with adequate anticoagulation and image-verified absence of neurological infarcts, patients with AF still have significantly greater cognitive impairment and a higher risk of dementia than matched patients without AF.1 In addition, there is an increased awareness of the association of AF with multiple forms of dementia, including Alzheimer’s disease, in the absence of stroke.1,7 Furthermore, many patients complain of transiently impaired cognition symptoms not explainable by focal, ischaemic injury. These patients describe a mental ‘fog’ or lack of mental ‘sharpness’ when in AF compared to normal sinus rhythm, which improves when normal sinus rhythm is restored. Therefore, other physiological changes caused by or associated with AF must occur.
In this review, we will discuss a prominent and developing hypothesis: AF contributes to cognitive decline and dementia through hypo-hyperperfusion events occurring during cardiac arrhythmias resulting in cerebral vascular dysfunction. We will summarise central nervous system (CNS) perfusion, measurement tools, and relationships between changes in perfusion and cognitive function. We will then compile the recent research that has examined brain perfusion during AF. Finally, we will discuss the critical areas for improvement necessary to draw crucial links between AF hypo-hyperperfusion events and cognitive dysfunction.
Atrial Fibrillation and Brain Health
There is an evolving understanding of the complex injury and disease patterns in the brains of patients with AF. A recent study of 1,737 patients with AF performed brain MRI at enrolment, which found large non-cortical or cortical infarcts in 22%, small non-cortical infarcts in 21%, microbleeds in 22%, and white matter lesions in 99% of patients.5 These data show that much vascular brain damage is unrelated to ischaemic injury. This spectrum of brain disease present at baseline in these patients highlights that most injuries will be missed if brain health primarily focuses on stroke prevention. Furthermore, these data also showed each injury type alone and, in aggregate, influenced performance on cognitive testing.2
Consider the most common baseline brain imaging findings in patients with AF, white matter lesions that were present in nearly all patients (99%). White matter lesions are areas in the brain with abnormal myelination. The most common risk factors for white matter disease are related to cardiovascular disease, such as age, hypertension, dyslipidaemia, diabetes, smoking, elevated inflammatory markers, etc.7 Considering the brain MRI findings in patients with AF, the broad range from overt ischaemic strokes to microvascular disease may represent the wide range of micro- and macrovascular disease present in these patients. The proposed mechanisms of vascular dysfunction secondary to AF hypo-hyperperfusion events are shown in Figure 1 and specifically highlight ischaemic injury, loss of adaptation to haemodynamic stress, and unmasked cerebral microvascular dysfunction as contributors to the vascular mechanism of AF-related cognitive decline.
AF and Vascular Disease
AF is traditionally considered a symptom of systemic vascular disease that results in elevated filling pressures, left ventricular systolic and diastolic dysfunction, and left atrial enlargement and scarring.9 However, there are data to suggest that arrhythmia also plays a role in the development and progression of micro- and macrovascular disease.10,11 The presence of AF is associated with elevated levels of CRP, interleukin, and other inflammatory markers that can increase oxidative stress and endothelial injury.12,13 AF is associated with the activation of the sympathetic nervous system, renin-angiotensin-aldosterone system, and impaired insulin resistance.14–16 These factors can further promote endothelial dysfunction and result in micro- and macroarterial disease. The presence of systemic vascular disease and the haemodynamic perturbations of AF can augment the severity of one another in these often comorbid disease states.14,15
AF as a Hypo-hyperperfusion Event
AF perfusion physiology is well described. During normal sinus rhythm, the ventricles have adequate time to fill with blood and perfuse end organs with stable pulsatility. During the irregularly irregular AF rhythm, diastolic and systolic timing become inconsistent. This creates varying diastolic filling times, with some long, allowing for a complete filling of the ventricles, increased preload, and increased cardiac output. However, other diastolic phases are short, limiting the time for adequate ventricular filling and decreasing cardiac output. One study showed a 17% drop in cardiac output during exercise in a patient with atrial fibrillation compared to normal sinus rhythm (NSR).17 Depending on the intrinsic cardiac rate, these filling times can be so abbreviated that symptoms of end-organ dysfunction can manifest acutely, such as shortness of breath, fatigue, or mental fog and cognitive dysfunction relevant to this review. The haemodynamic perturbations of the ventricles are worsened by the loss of atrial systole, which is progressively more critical with diastolic dysfunction, a common driver of AF genesis.
The result of the irregularly irregular AF rhythm is a series of hypo-hyperperfusion events in the microvasculature, which triggers vascular changes to prevent end-organ damage.18 In the long term, these vascular adaptations can develop into fulminant vascular disease.19 For example, flow-mediated dilation is impaired in patients with AF compared to sinus rhythm, which strongly indicates arterial endothelial dysfunction. Despite the left ventricle mounting a normal stroke volume, the cerebral parenchyma might experience hypoperfusion in the absence of normal flow-mediated dilation. Additionally, the impairment worsens as AF progresses from paroxysmal to persistent forms. Of interest, flow-mediated dilatation does improve after cardioversion and with catheter ablation to suggest some reversibility of the arrhythmia-mediated dysfunction.10,20,21 Additional markers of macro- and microvascular impairment have been observed in patients with AF compared to those in sinus rhythm, including hyperaemia pulse amplitude tonometry index, arterial pulse wave velocity and augmentation index, and reflecting conduit artery endothelial function.22,8,23,3 The multiple measures of vascular function corroborate the proposed understanding that AF manifests and contributes to macro- and microvascular dysfunction. As the AF burden increases, end-organ vascular beds are permanently altered and negatively impact the long-term stability of end-organ perfusion.
Furthermore, AF hypoperfusion could play a role in worsening vascular disease by enhanced oxidative stress. It has been shown that oxidative stress can worsen in situations of vascular hypoperfusion.12,13 Increased oxidative stress, especially in neuronal tissue, can have catastrophic effects, including mitochondrial failure, eNOS uncoupling, and neuronal cell death.12,13 This mechanism has been clearly linked to several forms of dementia, including Alzheimer’s disease.24 As a consequence, hypoperfusion during AF could also contribute to increased cellular oxidative stress leading to accelerated neuronal cell death, which as a mechanistic pathway linking both vascular and neuronal cell injury, and death substantiates the need for additional study to determine if early appropriate treatment many mitigate risk.
CNS Vascular Perfusion: Physiological Features and Measurement Techniques
Fundamentally, the brain is an ‘over-perfused’ organ capable of rapid adaptation to maintain function and health. Some estimates suggest the brain has upwards of 200% of the necessary blood flow required to maintain normal function.25,26 The most likely explanation for this over-perfusion is to protect brain function, prevent injury, and maintain central blood pressure. Additionally, brain tissue has a redundant vascular supply, i.e. multiple vessels from different main branches perfusing the same region of brain tissue.27 Finally, the brain vasculature is highly adaptable. In the case of malperfusion, brain vasculature can significantly constrict or dilate to support specific areas or global perfusion loss for extended periods.28,29
Non-invasive measurements of brain perfusion have been a mainstay of stroke management for over a decade. A suite of CT and MRI sequences examine specific tissue regions and the local perfusion. These methods are instrumental in identifying candidates for thrombolysis or thrombectomy in acute stroke management. However, most of the testing and validation of these sequences have been performed on patients with acute vessel blockage and small regional changes.30 Some studies have shown that incomplete obstruction leading to decreased regional brain perfusion is related to changes in cognitive function.31–34 These studies show promise for perfusion changes pertaining to cognitive function changes; however, few data sets show that decreased brain perfusion is directly related to cognitive decline. In one study that used MRI to assess cerebral blood flow and perfusion, patients with relatively low total brain perfusion were found to have significantly larger white matter lesions than their counterparts with normal cerebral blood flow. As white matter lesions have been correlated with risk of impaired cognitive function, there is clear benefit to avoiding hypoperfusion and optimising cerebral blood flow.35
One particularly intriguing cerebrovascular blood flow measurement technique is called cerebrovascular reserve blood flow (CVR).25,30,36 CVR is calculated by using two imaging phases. The first phase measures cerebral blood flow at baseline throughout the brain. The vasculature is then ‘challenged’ by administering a potent CNS vasodilator (commonly acetazolamide at high doses). The acetazolamide dilates the cerebral vessels by increasing the blood CO2 level and induces a local extracellular acidosis in the brain that triggers an increase in blood flow.25 After the drug has taken effect, the cerebral blood flow is again imaged and measured. Various imaging modalities can be used, but the most common is MRI because of its ubiquity and limited side effects, such as radiation exposure. CVR is then calculated in specific regions based on the change in blood flow between baseline and pharmacological stimulation. CVR is used to evaluate for ‘subclinical’ changes in brain vascular function before the onset of significant symptoms.36 Mechanistically, CVR is the best measurement tool for detecting cerebrovascular dysfunction that occurs during AF and can capture changes not detectable with traditional flow calculations.25,30,36
Changes in Brain Perfusion During AF
Despite vascular physiology that allows marked adaptation of the brain to preserve perfusion, recent data have shown that AF significantly impairs perfusion. One study compared 44 patients by assessing MRI perfusion sequences pre- and post-cardioversion to determine changes to brain perfusion in both AF and normal sinus rhythm. Specifically, they showed that at 10 weeks post-cardioversion, patients who remained in sinus rhythm had a 5% increase in cerebral blood flow compared to patients who did not convert to sinus rhythm. This study showed that restoration of normal sinus rhythm improves brain perfusion and that the effect persists after cardioversion.37
A recent clinical trial using similar brain perfusion imaging techniques assessed changes in cerebral blood flow and brain perfusion before and after catheter ablation for AF.38 Fifty-seven patients were enrolled and imaged using MRI perfusion imaging 1 day prior and 6 months after catheter ablation. They showed that in patients who underwent catheter ablation for AF and remained in sinus rhythm, cerebral blood flow increased by 39.26 ml/min compared to -34.86 ml/min in controls (p<0.01). Patients who successfully underwent cardiac ablation of AF treatment had an increase of 13.7% in cerebral blood flow. This study also examined regional brain volume changes pre- and post-AF ablation. The hippocampus has been noted to be the most susceptible to hypoxic injury. In this study, there were no changes to the hippocampal volume pre- and post-ablation (7.13 +/- 0.82 ml versus 7.17 +/- 0.83 ml [p=0.8]). These results are consistent with the proposed mechanism, given that ablation should correct the insult and thus halt the progression of hippocampal injury. It would be interesting to examine changes to the hippocampus in patients with persistent or permanent AF to determine if AF is a primary driver of structural changes. Unfortunately, cognitive testing was not performed on these patients; therefore, assessing the clinical significance of these changes in perfusion from AF catheter ablation is difficult.
Another study used a unique approach with near-infrared spectroscopy (NIRS) systems to evaluate blood flow in brain regions for patients in AF compared to matched controls.39 NIRS is an imaging technique that uses similar principles compared to pulse oximetry to measure the blood flow in specific brain regions. The protocol used a 52-channel machine placed on the patient’s forehead and around the temples to measure blood flow. The NIRS system measures the changes in oxyhaemoglobin to approximate blood flow. Furthermore, during the measurements, patients were asked to perform cognitive tasks. They showed a significant decrease in perfusion localised to the frontal region of the brain of patients in AF compared to patients who had no history of AF while performing specific cognitive tasks. Specifically, they showed that frontal brain activity was decreased by 37% in patients with AF (p=0.02) compared to age and comorbidity-matched controls. Furthermore, a subset of these patients in AF underwent catheter ablation and were re-imaged at 3 months post-ablation if they remained in sinus rhythm. They found that 62.5% of patients who underwent catheter ablation had increased blood flow to the frontal brain region and 75% to the temporal brain region.
The authors also investigated changes in depression or cognitive function with changes in cerebral blood flow. They found that improvement in perfusion of the frontal lobes was associated with improved depression scores (i.e. feeling less depressed) (R=-0.973, p=0.019), and improvement in perfusion to the temporal lobes was associated with improvement in cognitive function (R=0.0749, p=0.033). This study is one of the first to draw the connections between brain perfusion, specific domains of cognitive function, and AF pre- and post-return to normal sinus rhythm. These exciting results do come with caveats. The number of patients observed in the study was small (29 controls and 32 AF patients), and the number of patients who were ablated and maintained normal sinus rhythm was significantly smaller (8 total patients). However, the simplicity of the NIRS measurements and the initial positive results suggest that a large-scale evaluation could be easily achievable with relatively small research overhead, a previous limitation of advanced imaging techniques such as MRI or CT scans. Another interesting facet of this study is that patients performed a cognitive task while calculating the flow. Most studies have examined cerebral blood flow as a static event, i.e. no brain stimulation during imaging. However, this study incorporated set activities to stimulate perfusion. These results confirm the significant decrease in perfusion in patients with AF with or without cognitive stimulation.
Our group has also explored these mechanisms within a controlled experimental model.40 We assessed cerebral blood flow using MRI imaging in a previously validated model of AF in canines. Our imaging protocol focused not on direct measurements of cerebrovascular blood flow, but on cerebrovascular reserve (CVR) blood flow. This is a unique metric not used in previous AF CNS perfusion-related studies. Cerebrovascular reserve assesses the amount of vasodilation and increased perfusion available to the brain. We believe this is a more appropriate assessment of the perfusion and would produce similar changes requiring cognitive tasks during the examination. Furthermore, this approach highlights the vascular dysfunction secondary to prolonged AF. We acquired pre-AF induction imaging on animals and measured cerebral perfusion. AF was induced using chronic pacing for 6 months. Animals were imaged at baseline, 3-, and 6-month time points. Imaging was performed with animals cardioverted to normal sinus rhythm. At baseline, the average CVR was approximately 40% in the grey and white matter regions, compared to -17% at 3 months and -3% at 6 months. Measured ejection fraction (EF) from cardiac MRI at baseline was 36% (range 30–40%) and 31% (range 25–35%) at 6 months.
Interestingly, CVR was less at the 3-month scan (-17%) than at the 6-month scan (-3%). This suggests that there is a significant impact on the vascular perfusion of the brain that then becomes compensated over prolonged exposure but never returns to baseline levels. This resonates with patient anecdotes about initially being more symptomatic, regressing over time – an effect often attributed to hedonic adaptation may also have a pathophysiological basis. In this study, cognitive assessments on animals were not performed to understand how CVR relates to cognitive task performance. However, these data provide background for studies to examine cerebrovascular reserve in humans with AF and how these correlate explicitly with symptoms and cognitive function.
In summary, data suggest that AF causes significant haemodynamic stress on the CNS, which can be detected via changes to brain perfusion. These changes are caused by beat-to-beat variability with an irregular heart rhythm, which develops subsequent impaired diastolic filling. Impaired diastolic filing promotes acute and chronic stress responses and maladaptive consequences that lead to vascular dysfunction. These vascular dysfunctions collectively lead to cognitive decline and dementia in patients with uncorrected AF (Figure 2), and may even manifest when these patients are in sinus rhythm.
Potential Implications
The implications of these findings are enormous in the field of cardiac electrophysiology and specifically AF treatment. AF is the most common sustained cardiac arrhythmia, and the prevalence increases as the population ages, dependent and independent of higher traditional cardiovascular risk factors.41 Furthermore, the global rate of cognitive decline and dementia is projected to increase significantly in the following decades.4 Unfortunately, treatment modalities for addressing cognitive dysfunction directly, after disease manifestation, are minimal and have variable results. However, suppose these hypotheses regarding the critical role of brain perfusion and vascular maladaptation in patients with AF are confirmed. In that case, treatment of AF could become a mainstay for dementia and cognitive decline prevention in these patients.
Furthermore, these findings would call into question our current paradigms for AF treatment. Current treatment of AF is focused mainly on three main strategies: anticoagulation to decrease stroke risk, rate control to preserve perfusion and prevent cardiomyopathy, and rhythm control primarily to improve quality of life and/or symptoms. Almost all patients are advised to be anticoagulated to reduce stroke risk; however, the following option to manage perfusion is still unclear. Initial studies that explored the role of rhythm versus rate control as it related to brain health (stroke) and mortality found no advantage to either strategy.42,43 However, in both trials, the conclusions were drawn without considering when AF developed and how treatment delays may negate the value of early rhythm control approaches. Additionally, stroke rates increased as anticoagulation use declined in these trials. In a recent trial that only included patients in which AF was present for 1 year or less, rhythm control achieved through various methods coupled with sustained use of anticoagulation significantly improved the risk of stroke, acute coronary syndrome, and mortality.44 There appears to be an opportunity to improve cognitive dysfunction and dementia risk in patients with AF drawing from the concept of earlier treatment and perhaps upstream preventative therapy. If the hypothesis is that AF causes continuous malperfusion and results in vascular maladaptation that affects cognitive function and brain health, then the ideal intervention would be early rhythm control via pharmacological or surgical approaches. This would be a massive change in the current management approach to AF. Before these changes come to fruition, further research and understanding must exist. It should be noted that silent cerebral lesions have been detected post catheter ablation via MRI imaging ranging from 4 to 39%, and vary by ablation technology used and procedural techniques.45–47 This procedural risk is offset somewhat in that the risk of long-term development of cerebral infarcts in patients who underwent catheter ablation for AF rhythm control is lower than in patients who did not receive catheter ablation.48 However, it is critical to realise that there is an actual risk of brain injury during ablation and that risk is offset by the long-term value of sinus rhythm and AF-related risk factor reduction.
Finally, early intervention will be crucial. As we have shown in our canine model of brain perfusion, and as supported by clinical data of other AF-related outcomes, perfusion seems to be most significantly affected at the beginning of the disease, with adaptations occurring at approximately 6 months.44,49 Therefore, most brain damage may occur early in the disease process when the tissue is not well perfused initially. This untoward possibility is clearly illustrated in the SWISS-AF study in which brain injury was present essentially in all patients with AF at the time of study enrolment.2 This would suggest that early AF intervention is vital to preserving brain tissue despite the long-term adaptations of brain vasculature. However, as AF diagnosis continues to evolve towards direct-to-consumer products such as smartwatches and mobile telemetry, even earlier diagnosis and treatment are possible.50 Studies such as the Heartline study (NCT04276441) are underway to determine to what magnitude these devices impact decisions and if these tools, coupled with education, improve the use of guideline-directed therapies.
Completing the Puzzle: Future Investigations
The first and most important piece missing is the link between malperfusion events occurring during AF and changes in cognitive function. As we have illustrated, studies have shown that AF causes decreased overall brain perfusion, which can be rectified in normal sinus rhythm. Other studies have also shown, albeit outside of the context of AF, that poor brain perfusion leads to cognitive dysfunction and, over time, dementia.34 However, we are missing a comprehensive study that links the presence or burden of AF, brain perfusion, and cognitive dysfunction. The necessary future study would select patients with AF who complete baseline cognitive and brain perfusion studies. The patients would then be cardioverted to normal sinus rhythm, and the tests would be repeated.
Another critical discussion among the community is the need for robust and standardised brain perfusion measurements. As we have discussed, there are many ways to measure brain perfusion with MRI, CT, NIRS, and others. However, there is no consensus on the ideal measurement tool. Furthermore, these perfusion measurement systems have been designed, tested and implemented on patients with normal sinus rhythm. In some circumstances, there are assumptions inherent to the method that require patients to be in a normal cardiac rhythm. Therefore, further examination and testing must be performed to identify the ideal measurement technique and standardise the approach for the community.
These studies would have many different parameters to adjust and study the short- and long-term implications of AF on cognitive function, such as time in sinus rhythm, time in AF, relative cognitive deficits, and other clinical comorbidities. These parameters and features will need to be independently tested and validated over large patient populations to understand better how AF changes cognitive function.
Future work could also focus on experimental approaches in animal models to solidify mechanistic changes to brain function and controlled episodes of AF. Cognitive function and cognitive assessments are significantly more challenging to perform, but histological and functional imaging may provide unique insights into the complicated mechanism.
Other studies need to examine how the AF burden affects cognitive changes. Specifically, patients with persistent or permanent AF may report fewer cognitive dysfunction symptoms potentially due to the brain’s vascular physiology that has adapted to provide the necessary perfusion to the CNS. Studies could be undertaken to understand how patients with paroxysmal AF report cognitive symptoms compared to patients in persistent or long-standing persistent subtypes. These studies could identify key long-term adaptations that are not available in the short term, much as our preliminary data suggests.
Another avenue of investigation should include the recoverability of the brain and, if incomplete, identify a mechanism that can be targeted to improve the likelihood of recovery. AF can be found incidentally in many patients; therefore, the length of time a patient is in AF will be unknown. Some studies have shown patients can be in AF for decades with little to no awareness. The adaptive changes of the brain vasculature may have permanently altered and may increase the risk for other vascular events if the patient is converted to normal sinus rhythm. Furthermore, if normal sinus rhythm is realised, should we expect a recovery of cognitive function or a slowing of the progression of cognitive decline?
Answering these key questions would open the door for future improvements and further understanding of the unique interaction of AF and cognitive decline. Furthermore, this would integrally link two of the most important human organs.
Clinical Perspective
- AF is associated with an elevated risk of cognitive decline and dementia.
- AF results in hypo- and hyperperfusion events in the brain that can impact microvascular function and result in end-organ dysfunction and disease.
- AF can impair cerebrovascular reserve and reduce the ability of brain compensation to the haemodynamic stress of the arrhythmia.
- Rhythm control strategies may improve cognitive dysfunction and dementia risk in patients with AF although randomised prospective trial data are lacking.