Autoimmune rheumatic diseases (ARD) encompass a heterogeneous group of diseases affecting not only the musculoskeletal system but also multiple organs or systems.1 Among them, the heart and the cardiovascular system may be affected through different pathophysiological mechanisms including myocardial inflammation and/or fibrosis, vasculitis, thromboembolic events or premature atherosclerosis. All these mechanisms can lead to an increased incidence of altered automaticity and reentry phenomena in patients with ARD.2–5
Conduction disorders occur mainly during flares of ARD and are in general more frequent than rhythm disturbances.6 Rhythm disorders in patients with ARD have different, and not fully understood, underlying pathophysiological mechanisms with myocardial inflammation and fibrosis being the most important ones. Inflammatory processes and oxidative stress lead to cardiomyocyte necrosis with subsequent electrical and structural remodelling. Chronic inflammation leads to autonomic dysfunction, namely sympathetic overactivation and decreased parasympathetic function. Autoantibody-mediated and drug-induced arrhythmias are also frequently observed among patients with ARD.7
Although all heart structures can be affected in patients with systemic ARD, in this review we will focus on rhythm and conduction disturbances occurring in patients with systemic ARD, such as systemic lupus erythematosus (SLE), systemic sclerosis (SSc), rheumatoid arthritis (RA), idiopathic inflammatory myopathies (IIM), and small-size vessel vasculitis and in particular the anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV). The relative prevalence of the different types of arrhythmias in patients with ARD (Table 1) has emerged from a limited number of small clinical studies.8
Supraventricular Arrhythmias and Tachyarrhythmias in ARD
Supraventricular arrhythmias (SA) are commonly observed in patients with ARD.9 The reported frequency of SA is variable, depending on diagnostic methods and patient selection.10
Systemic Lupus Erythematosus
SLE is a prototype autoimmune disease characterised by a plethora of autoantibodies directed against circulating, cytoplasmic or nuclear autoantigens and a wide spectrum of clinical, laboratory and histopathological manifestations from the affected organs including the joints, skin, kidneys, lungs, nervous system and the heart.11 In some patients, the disease runs an indolent course, while in others it can threaten the function of the affected organs and even the patient’s life.11
The underlying mechanisms of SA in SLE have not been extensively investigated and are probably numerous. Sinus tachycardia, AF and atrial ectopic beats are the most frequent arrhythmias seen in patients with SLE.9–14 SA are often transient and may be related to lupus myocarditis and exacerbations of SLE with fever, volume depletion and congestive failure.
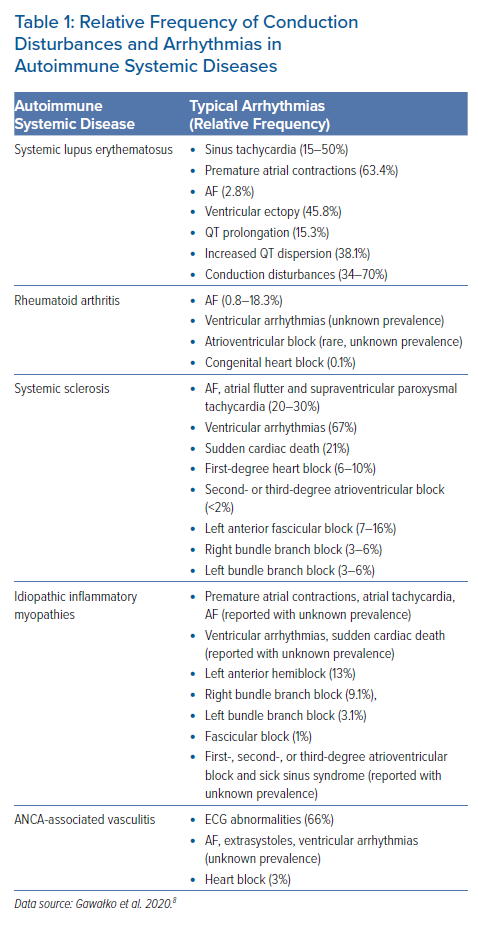
In a study by Texeira et al. that included 317 patients with SLE, Holter monitoring abnormalities were observed in about 85% of patients with SLE, including supraventricular ectopy (63.4%), bradycardia (31.7%), atrial tachycardia (15.5%) and AF (2.8%).15
In other studies, sinus tachycardia was found to occur in 50% of patients with SLE and was the only cardiac manifestation of SLE, which resolves with corticosteroid treatment.14 Vasculitis-induced myocardial fibrosis or accelerated coronary atherosclerosis have been proposed as underlying mechanisms of SA in SLE through ectopic automatism, triggered activity or reentry.14–17
Systemic Sclerosis
Systemic sclerosis (SSc) is a rare connective tissue disorder of unknown and complex pathogenesis, its main feature being the excessive production and accumulation of collagen leading to fibrosis of the affected organs.11 Scleroderma can be divided into two forms – localised scleroderma or systemic sclerosis – based on clinical and serological criteria. These two forms can further be classified as either limited cutaneous SSc or diffuse cutaneous SSc.
Localised scleroderma is a disease of the skin and subcutaneous tissue and it is not associated with internal organ involvement or with increased mortality. However, SSc is associated with specific autoantibody positivity, systemic manifestations, internal organ involvement and increased mortality. The organs most frequently affected by scleroderma are the skin, gastrointestinal tract, lungs, kidneys, skeletal muscle and heart.11
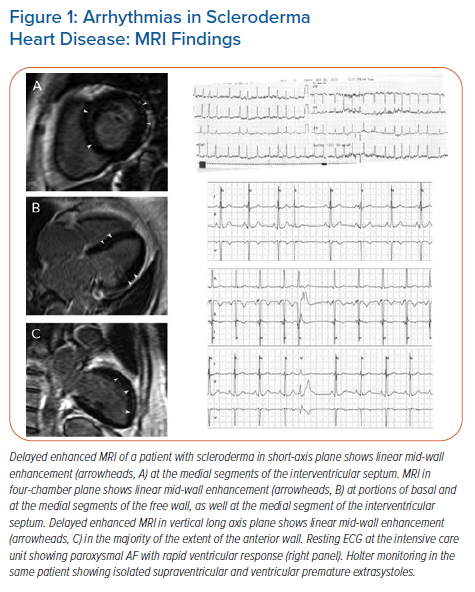
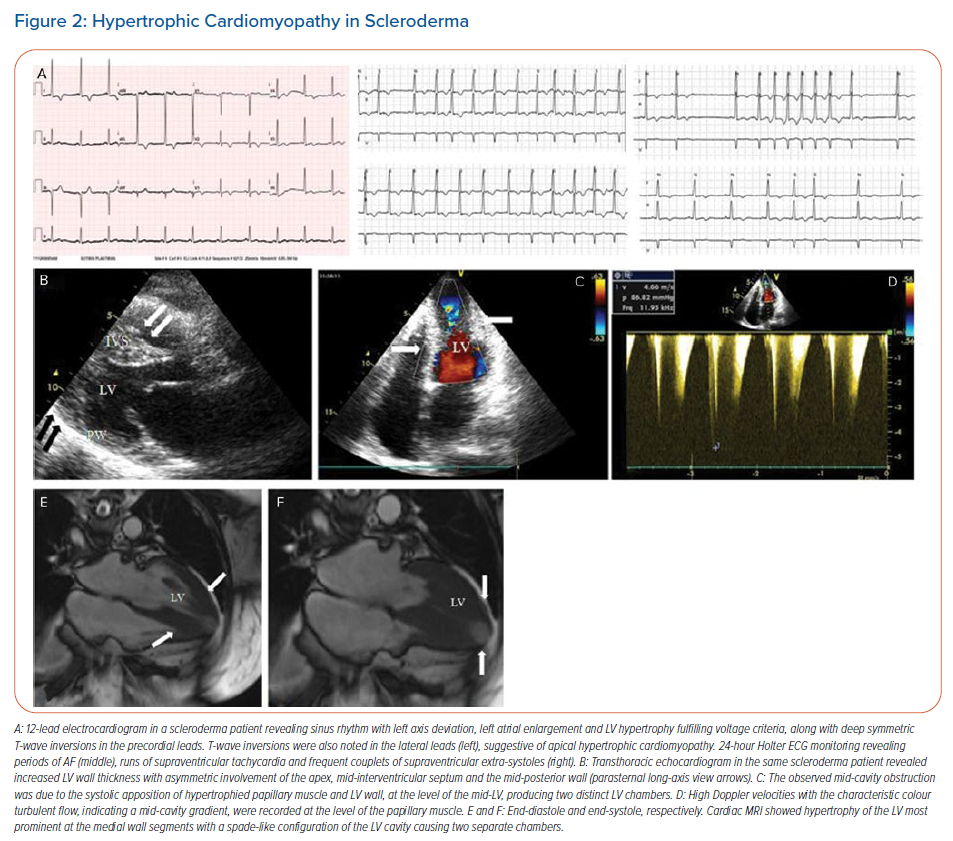
SA are frequently seen in patients with SSc as a result of focal myocardial fibrosis (Figures 1 and 2).18–24 AF, atrial flutter and supraventricular paroxysmal tachycardia have been reported in 20–30% of patients.2,23 Patients with SSc have been found to have a higher mean heart rate (81 ± 11 BPM), with more cases of sinus tachycardia reported in limited SSc than in diffuse SSc.19,21–23 Myocardial fibrosis that disrupts the normal electrical connectivity of cardiac tissue, left atrial dilation secondary to a higher prevalence of pulmonary hypertension, and more severe mitral and tricuspid regurgitation, have been proposed as possible underlying mechanisms for SA in SSc.19
Atrial fibrosis, a common feature of clinical AF and a hallmark of arrhythmogenic structural remodelling, is directly related to the degree of left atrial (LA) dilation that accompanies left ventricular (LV) diastolic dysfunction in patients with SSc. In those with LV diastolic dysfunction, LA myocyte hypertrophy, LA areas of fibrosis, elevated LA pressure and LA dilation constitute the basis for the occurrence of AF and have been associated with a poor prognosis.
Rheumatoid arthritis (RA) is a systemic inflammatory disease affecting mainly the synovial tissue of small and large joints. Autoantibodies to immunoglobulins (rheumatoid factors) and citrullinated proteins are frequently found in the patients’ sera and the pleural cavity, the lung parenchyma and the heart can be affected in about one-quarter of the patients.11 The incidence of AF in patients with RA is 40% higher than in the general population and can occur any time during the disease course, although it can be the first disease manifestation.25 Although the pathophysiology of AF in RA is complex and poorly understood, several lines of evidence support that systemic inflammation causing increased circulating concentrations of inflammatory proteins, ischaemic heart disease and heart failure are important factors for the initiation and recurrence of AF in this patient group.25–27
It has been shown that the rate of successful cardioversion is lower in patients with RA who have AF and high inflammatory burden with persistently increased serum inflammatory indices.27–29 Increased P wave dispersion in electrocardiography, which is considered to be a predictor of AF, also occurs more frequently in patients with RA and seems to be highly associated with the level of systemic inflammation.30
Autonomic nervous system (ANS) dysfunction due to the neurotoxic effect of chronic systemic inflammatory process associated with RA and the side-effects of therapeutic agents, is evident in about 60% of patients with RA. ANS dysfunction is considered a possible pathogenetic cause of SA.31 The main pattern of ANS deregulation is impairment of cardiovascular reflexes and altered heart rate variability, indicative of reduced cardiac parasympathetic activity and elevated cardiac sympathetic activity manifesting as atrial ectopic beats, impaired heart rate control and inappropriate atrial tachycardia.32
Idiopathic Inflammatory Myopathies
IIM are rare autoimmune systemic disorders characterised primarily by muscle (polymyositis) and/or skin (dermatomyositis) inflammation.10,11 Lung and cardiac involvement can be seen in this patient population and they affect survival.33–36 Recent non-invasive diagnostic modalities suggest that cardiac involvement in patients with IIM ranges from 9–72% based on different selection criteria for patients in studies.37 Myocarditis, focal fibrosis, vasculitis, intimal proliferation or medial sclerosis of vessels have been proposed as possible mechanisms of abnormal electrical activity based on abnormal automaticity.38 Studies of patients with IIM based on ECG and Holter monitoring showed frequent premature atrial beats, atrial tachycardia, paroxysmal AF and multiple focal right atrial tachycardia mainly caused by abnormal automaticity rather than triggered activity or reentry.38,39
Anti-neutrophil Cytoplasmic Antibody-associated Vasculitides
AAV are a group of disorders characterised by inflammation of blood vessels, endothelial injury and tissue damage. Although any tissue can be involved in AAV, the upper and lower respiratory tract, the kidneys, the skin and the peripheral nervous system are most commonly and severely affected more frequently rather than the central nervous system.11
The three types of AAV are granulomatosis with polyangiitis (GPA; previously known as Wegener’s granulomatosis), microscopic polyangiitis (MPA) and eosinophilic GPA (EGPA; previously known as Churg–Strauss syndrome). Autoantibodies to the neutrophil proteins leukocyte proteinase 3 (PR3-ANCA) or myeloperoxidase (MPO-ANCA) are typically present, depending on the type of AAV.11 Clinically significant cardiac involvement is a rare complication in systemic AAV, but it can be life-threatening.40,41
Since many cardiac manifestations are clinically silent, at least during their early stages, heart function should be systematically evaluated by ECG and echocardiography. AAV is significantly associated with AF which confers independently worse survival rates.
Several plausible explanations for the association between vasculitis and AF have been proposed; blood vessel wall inflammation results in increased arterial stiffness and decreased vascular distensibility leading to vascular damage and end-organ ischaemia, all of which are considered important mechanisms for atrial tachyarrhythmias in this particular group of patients.41–43 Alteration in microvascular circulation is another possible haemodynamic mechanism.41 Delay in atrial emptying due to impaired diastolic distensibility that increases pressure and wall stretch within the atria and pulmonary veins is also an important mechanism for altered atrial electrical properties that promotes AF development.
Ventricular Arrhythmias in ARD
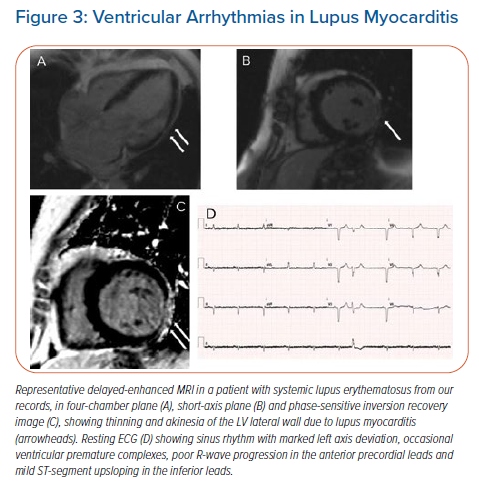
Although supraventricular tachycardia is the most common finding in SLE, ventricular arrhythmias (VA) can occur and are mainly due to coronary artery disease (CAD).44,45 VA in SLE can also be the result of inflammatory cardiomyopathy, causing structural and electrical heart disease which may have a serious impact on the patient’s outcome.46,47 Acute myocarditis in SLE may present with ventricular tachycardia (VT) as an initial manifestation.48 Figure 3 is a representative case of lupus myocarditis detected with cardiac magnetic resonance (CMR) in our department presenting with frequent ventricular extrasystoles.
Dilated cardiomyopathy with normal coronary arteries can be seen in patients with SLE.49 Although VA are infrequent among these patients, the Systemic Lupus International Collaborating Clinics Registry revealed a high prevalence of QT prolongation (15.3%) and increased QT dispersion (38.1%), both of which are recognised as independent risk factors for the development of complex VA.46 Myocardial scar due to ischaemic or non-ischaemic heart disease is the main cause of VA in SLE.
Myocardial inflammation, either isolated or as part of the systemic inflammation, is another important cause of VA in SLE.46–49 In addition, chronic use of antimalarial drugs, which are a common therapeutic modality for SLE, may also lead to VT.49 Sympathetic hyperactivity as shown by the elevated norepinephrine levels can play an aetiological factor for VA.6
Previous studies have reported a decrease in parasympathetic activity due to the autoantibodies against the Sjögren syndrome-related antigen A (Ro/SSA) and M3 muscarinic receptors.15 The presence of anti-Ro/SSA antibodies in patients with SLE have been associated with prolongation of the corrected QT interval (QTc).49 Lazzerini et al. reported that in addition to the high prevalence of QTc prolongation, anti-Ro/SSA-positive patients also have reduced heart rate variability and a high incidence of ventricular late potentials.49
VA are common among patients with asymptomatic SSc and may be associated with poor outcome.2 Patchy or linear myocardial fibrosis provides an ideal substrate for VA dependent on reentrant circuits (Figure 1). VA in patients with SSc can also develop due to overproduction of anti-β1-adrenergic receptor autoantibodies which lead to autonomic dysfunction. This usually precedes the development of myocardial fibrosis.50,51
VA have been demonstrated in 67% and non-sustained ventricular tachycardia (VT) in 7–13% of unselected patients with SSc.23,50 Patients with frequent premature ventricular contractions had 50% mortality during 33 months of follow-up, in contrast to 8% in SSc patients without frequent ectopy.51 Bulkley et al. noted sudden cardiac death (SCD) in 21% of patients with SSc, in contrast to the study by Lee et al. in which no SCD was observed in 61 deaths of 275 patients with SSc.51,52 In the study by Follansbee et al., SCD was confirmed in 5% of the 1,258 patients with SSc.53 VA and SCD occur in patients with both skeletal muscle disease and myocardial involvement.
In our study, 63% of patients with SSc had premature ventricular contractions, while 10.5% had non-sustained VT.20 SSc patients with VA documented by Holter were more likely to have pulmonary hypertension, decreased LV ejection fraction (LVEF), increased RV diameter and LA distention documented by Doppler echocardiography; they also had a greater number of enhancing myocardial segments suggestive of myocardial fibrosis in delayed enhanced CMR.20
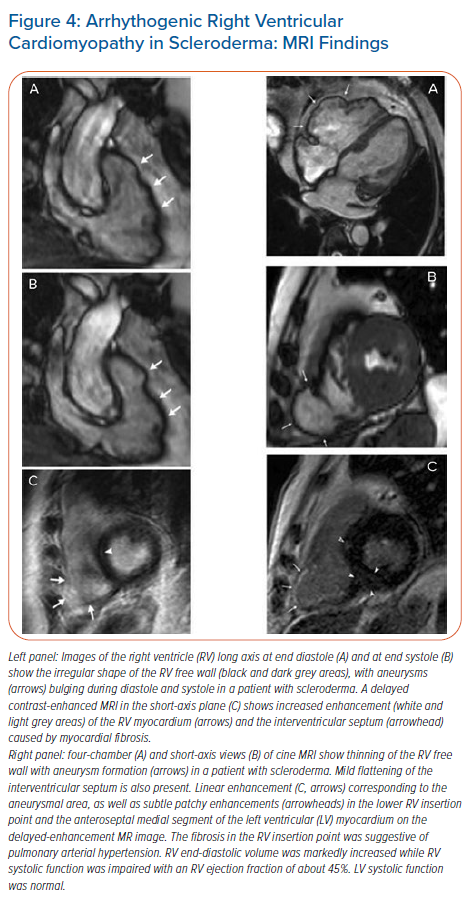
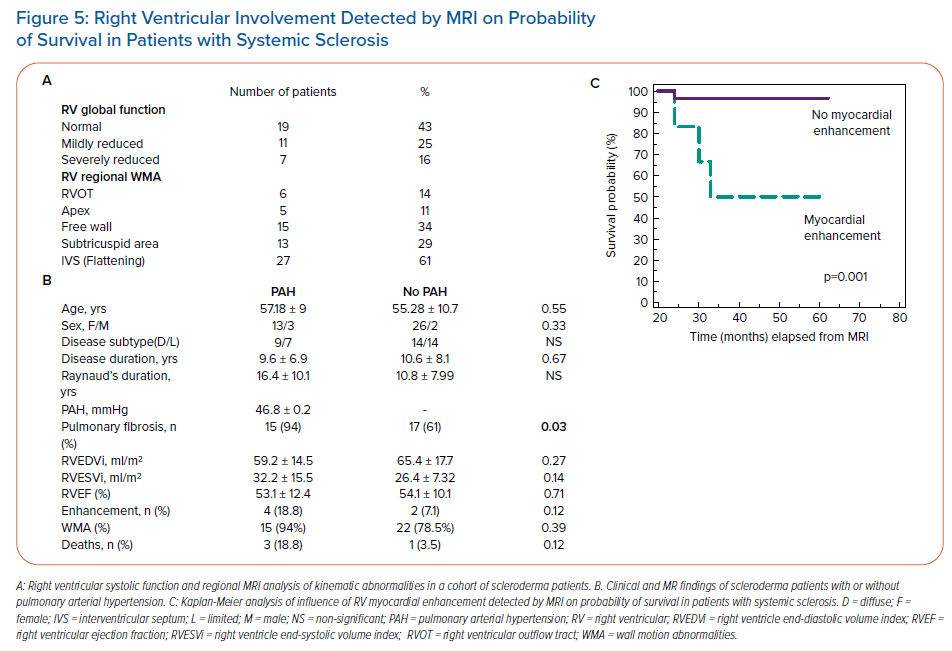
Involvement of the LV basal infero-septal segment and right ventricular (RV) dysfunction with hypokinetic and dyskinetic areas involving the RV free wall resembling arrhythmogenic RV dysplasia, as well as aneurysms of the RV and RV outflow tract were more commonly seen in patients with SSc with malignant VA than in those without arrhythmias (Figure 4). The extent to which this indicates a causal relationship requires further electrophysiological confirmation. In our unpublished data on 44 consecutive SSc patients with no history of ischaemic cardiomyopathy or risk factors for CAD, delayed enhanced CMR revealed functional and morphological abnormalities in the majority of patients (84%) (Figure 5). Myocardial fibrosis in the RV myocardium which was detected in a small percentage of patients with SSc (6%) by delayed enhanced CMR was associated with poor survival due to the high incidence of malignant VA (Figure 5).
The most common cause of SCD in patients with RA is atherosclerotic CAD that may lead to acute coronary syndrome and VT.2 Although the underlying mechanisms accounting for the pro-arrhythmogenic substrate in RA are probably intricate, the leading role seems to be played by chronic systemic inflammatory activation as it is able to promote arrhythmias both indirectly by accelerating the development of ischaemic heart disease and congestive heart failure, and directly by affecting cardiac electrophysiology.6 Furthermore, there is evidence that the inflammatory cytokines, mainly tumour necrosis factor (TNF)-α, interleukin-1 and interleukin-6, can modulate the expression and function of ion channels both by directly acting on cardiomyocytes.6
VT has also been described in patients with RA as a consequence of giant cell myocarditis, a rare but fatal cardiac disease complication characterised by degeneration and necrosis of myocardial fibres.54 VT has also been described in patients with RA related to treatment with methotrexate and infliximab.55,56 Increased sympathetic and decreased parasympathetic activity can play a crucial role in the development of VT in patients with RA.57
VT and SCD have also been described in people with IIM, although their incidence has not been precisely defined.58–60 Cardiac involvement with histological evidence of myocarditis is well documented in patients with IIM and may be the most usual cause of VA. The histopathology of the myocarditis resembles inflammation in the skeletal muscles including active myocarditis, focal fibrosis, vasculitis, intimal proliferation and medial sclerosis of vessels.
VA triggered by active vasculitis is uncommonly observed and rarely reported. In AAV infrequently, less than 10%, any cardiac tissue can be affected, with varied clinicopathological syndromes including pericarditis, myocarditis, coronary arteritis, valvulopathy and intracavitary cardiac thrombosis all of which can lead to VA.40
Conduction Disturbances in ARD
Conduction defects have been frequently diagnosed in patients with SLE (34–70%) and may be the result of active or past myocarditis.17 First-degree heart block may be seen and is often transient. Higher degrees of heart block are unusual in adults and are associated with the presence in the patient’s serum of anti-U1-nuclear ribonuclear protein antibodies, but not with anti-Ro/SSA or anti-La/SSB antibodies, as in the case of newborns of mothers with these autoantibodies.6
Neonatal lupus is a rare syndrome caused by the trans-placental passage of autoantibodies from mothers positive to anti-Ro/SSA (and/or anti-La/SSB) to their newborns. About 3% of infants whose mothers are antibody-positive develop complete heart block.61,62 Autopsy studies have revealed focal inflammatory cell infiltrates or, more often, fibrous scarring of the conduction system.61,62 Small vessel vasculitis and the infiltration by fibrous or granulation tissue are major causes of the dysfunction of sinus or atrioventricular (AV) nodes in SLE. Conduction abnormalities in SLE may regress when the disease is in remission.
Anti-Ro/SSA antibodies in adults with SLE may be associated with an increased likelihood of a prolonged QTc, which has been shown in patients without SLE to be a risk factor for VA and SCD.63–65 The clinical significance of this observation is unclear, given the low threshold (QTc ≥440 msec) used to define prolongation in these studies. However, patients with anti-Ro/SSA antibodies may benefit from having ECGs and receiving appropriate counselling if a prolonged QTc is detected.
Conduction system disease is common in patients with SSc and is present in about 25% of patients with SSc at the resting ECG.66–68 It is likely to be the result of myocardial and conduction system fibrosis.20,68 Most frequent conduction abnormalities are first-degree heart block (6–10%), left anterior fascicular block (7–16%), right (3–6%) and left (3–6%) bundle branch block and non-specific intra-ventricular conduction defects (2–3%).21 Second- or third-degree AV block is uncommon (<2%).66–68 Dysfunctional AV node due to fibrotic changes and a linear pattern of myocardial fibrosis with sparing of the subendocardial layer have been proposed as mechanisms responsible for conduction system disorders in patients with SSc.21,66
AV block is rare in patients with RA, but usually complete.69–71 Ahern et al. described congenital complete heart block in 0.1% of RA patients, especially in women, and concluded that it is more common in patients with subcutaneous nodules.69 Rheumatoid granuloma, CAD and non-specific inflammatory lesions are considered to be responsible for conduction disturbances in RA patients.70,71
Several types of conduction abnormalities are detectable in patients with IIM, including bundle branch block, fascicular block, first-, second- and third-degree AV block and sick sinus syndrome.33,72,73 Complete AV block is extremely rare.72 Direct involvement of the conduction system either due to myositis with contraction band necrosis or due to focal myocardial fibrosis (as a final result of myocarditis or myocardial ischaemia related to coronary vasculitis) have been proposed as possible causal mechanisms.73
In an electrophysiology study in four patients with IIM and bifascicular block, the site of block was localised distally to the His bundle suggesting that the conduction disturbances observed in this particular group of patients are mainly related to a direct involvement of the intraventricular conduction system.74
ANCA vasculitis has been associated with bundle branch blocks and all grades of heart block. These are believed to be from granulomatous inflammation involving the AV node or the bundle of His.75 While cardiac involvement is not usual in GPA, conduction abnormalities and accelerated atherosclerosis may occur during the disease course.76,77
Diagnostic Algorithm of Cardiac Arrhythmias in Patients with ARD
It is of great importance that an early and accurate diagnosis of cardiac arrhythmias and conduction disturbances is established in patients with ARD, given the fact that the heart involvement can be subclinical.1,2,7,19,78–86 These manifestations in this patient group should be the object of a careful investigation by rheumatologists and cardiologists for all patients with ARD aiming to prevent serious complications including sudden cardiac death.
Classic and newer diagnostic modalities are useful for assessing cardiac function and detecting myocardial involvement and CAD, which are the major substrate for arrhythmias and conduction disturbances in patients with ARD. Clinical, 12-lead electrocardiogram and conventional transthoracic echocardiographic evaluation is the first approach. Newer echocardiographic modalities including 2D/3D speckle-tracking imaging and global longitudinal strain should be used for the early diagnosis of global and regional kinetic disorders of the ventricles, diastolic dysfunction, LV hypertrophy and valvulopathies. However, while a normal echocardiography cannot rule out heart involvement in patients with ARD, PET and CMR can provide reliable diagnostic information about inflammation and scar by performing tissue characterisation.
While the proposed diagnostic algorithm for autoimmune inflammatory cardiomyopathy using PET includes non-ischaemic cardiomyopathy with LVEF <50%, documented monomorphic/polymorphic VT, ventricular fibrillation or frequent premature ventricular contractions and patchy focal or focal on diffuse fluorodeoxyglucose uptake on PET imaging, there is no specific algorithm for arrhythmia-induced cardiomyopathies using CMR.
However, an expert consensus proposed that CMR provides strong evidence of myocardial inflammation with increasing specificity, if the CMR scan demonstrates the combination of myocardial oedema with other CMR markers of inflammatory myocardial injury.87 This is based on at least one T2-based criterion including global or regional increase of myocardial T2 relaxation time or an increased signal intensity in T2-weighted CMR images, with at least one T1-based criterion such as increased myocardial T1, extracellular volume, or late gadolinium enhancement. CMR is able to provide a more realistic and more accurate visualisation of the ventricles than other conventional imaging methods.
Endomyocardial biopsy is considered the gold standard to diagnose myocarditis and/or inflammatory cardiomyopathy. However, it is an invasive method with excellent specificity but poor sensitivity due to sampling error given the patchy distribution of myocardial involvement in the majority of patients with ARD and the lack of agreement between specialists regarding interpretation of specimens.
Coronary angiography is the gold standard for the diagnosis of CAD. Using 24/48-hour Holter monitoring of cardiac rhythm can show specific and nonspecific findings, including any type of arrhythmias, changes of PQ and ST interval, prolongation of QRS complex, rhythm disorders and the presence of Q waves. Electrophysiology studies may be warranted to evaluate persistent palpitations, presyncope or syncope and complex supraventricular and ventricular extrasystoles as assessed in Holter studies in patients with ARD.
Treatment
Optimal immunosuppressive therapy of the underlying systemic disease and management of the arrhythmias based on the international treatment guidelines and evidence-based medicine with respect to the special feature of each disease remains the cornerstone of the correct approach in these patients.85,86 Unfortunately, there are few randomised controlled trials evaluating anti-arrhythmic therapies specifically for use in patients with ARD. Therefore, therapy should be tailored for each individual patient. Drug selection should be based on their electrophysiological effects and the type of arrhythmia. Non-dihydropyridine calcium channel blockers are preferred for the treatment of SA and may improve cardiac perfusion and ventricular function, potentially reducing the burden of arrhythmias.88,89
Digoxin can be used to decrease ventricular response in AF and in end-stage heart failure but is contraindicated in those patients with impaired renal function. The treatment of sinus tachycardia and supraventricular and ventricular extrasystoles is generally carried out by β-blockers, taking into account that they may worsen the symptomatology of severe Raynaud’s phenomenon. In these patients, a cardioselective β-blocker, such as bisoprolol, metoprolol and nebivolol, appears to be safe. Amiodarone should be avoided in patients with ARD particularly in those with interstitial lung disease.
Medically intractable life-threatening VT are treated with ICDs. These have been proven to decrease mortality in patients with dilated cardiomyopathy and are regarded as a treatment of choice for patients with heart failure and advanced LV dysfunction in combination with resynchronisation pacing.90,91 The need for ICDs should be carefully evaluated in patients with ARD with proven malignant VA and it will often require further electrophysiological confirmation.
Radiofrequency ablation is an effective approach to many types of arrhythmias.92–98 The role of radiofrequency ablation in patients with ARD has not yet been well evaluated but seems to be promising.
Catheter ablation seems to be safe in drug-resistant AF in patients with SLE and RA.2,99,100 This approach might be considered as the first-line therapy in patients with AF and systemic disorders, especially in those patients with ARD in whom the use of antiarrhythmic drugs is either contraindicated, scarcely effective, or associated with more adverse effects. Recurrence or persistence of AF after ablation has been attributed to peri-procedural inflammation. Thus, therapies targeting the procedure-related inflammation may reduce the risk of AF recurrence.99–101
Catheter ablation for atrial tachyarrhythmias, refractory premature ventricular contractions and ventricular tachycardia has been successfully applied in patients with IIM SSc and RA.38,102–105 ARD patients considered for RF catheter ablation for AF or VT are those with symptomatic, sustained, monomorphic VT when these are drug resistant, the patient is drug intolerant or does not desire long-term drug therapy.
Pacemaker implantation is the method of choice for the treatment of complete heart block and other serious conduction abnormalities. Sophisticated pacing modalities and programmability as well as low-energy circuity and new battery designs have increased device longevity and enabled wide clinical application.
Conclusion
Early and accurate diagnosis of heart involvement and rhythm disorders in patients with ARD is crucial and affects their overall mortality. Mounting evidence indicates that the increased risk of arrhythmias in patients with ARD is the combined result of the increased prevalence of structural changes (fibrosis) and electrical changes (gap junction impairment and intracellular calcium-handling abnormalities) caused by inflammatory cytokines, particularly tumour necrosis factor α, interleukin-6 and 1, and C-reactive protein. These phenomena promote arrhythmogenesis, by both conduction slowing and increasing ectopic activity, thus impairing the homogeneity of impulse propagation throughout the cardiac tissue.104,105
Given the complex nature and course of these diseases, anti-arrhythmic treatment should be based on international guidelines. However, current guidelines contain scant information for the treatment of cardiac arrhythmias in patients with ARD with mainly class C recommendations.104–106 Therefore, there is a need to update guidelines in collaboration with cardiologists and rheumatologists with simultaneous conduction of a large-scale registry that could improve the treatment of patients with ARD.
Understanding the mechanisms producing arrhythmias in ARD and combining newer diagnostic modalities that will guide new therapeutic strategies would probably improve the quality of life and the survival of patients. Electrophysiology studies may also be warranted to evaluate persistent palpitations, presyncope or syncope and complex supraventricular and ventricular extrasystoles as assessed by Holter studies in patients with ARD.
Even after the diagnosis of the arrhythmia, the electrophysiological evaluation of the arrhythmia substrate can be performed in the electrophysiology laboratory using 3D mapping systems. A detailed voltage mapping can reveal areas with low potential due to extensive fibrosis involved in reentrant circuits in the atria and ventricles.
Current research is focused on improving mapping techniques, developing new imaging modalities, creating new catheter designs for enhanced RF energy delivery and evaluating new energy sources for catheter ablation. Together, these efforts will undoubtedly extend the indications and improve the efficacy of catheter ablation. The integration of CMR data sets in 3D mapping systems could also greatly facilitate electrophysiology procedures.
Further technical and procedural improvements are warranted to bring these techniques to broader clinical practice for patients with ARD.
Clinical Perspective
- Autoimmune rheumatic disorders (ARD) are inflammatory conditions frequently affecting the blood vessels and the heart.
- Symptoms of arrhythmias or conduction defects are frequently reported in patients with ARD.
- The exact prevalence of cardiac arrhythmias in ARD is unclear, due to the small size and heterogeneity of patient populations.
- Rhythm disorders may have several origins related to primary heart involvement, coronary artery disease, pericardial disease, valvulopathies or pulmonary arterial hypertension and may increase morbidity and mortality.
- Further prospective large-scale studies are warranted to explore the pathogenesis and to improve the early diagnosis of cardiac rhythm disorders in patients with ARD.