AF Burden and Multi-pronged Approaches to Management
AF is the most common cardiac arrhythmia and is estimated to affect more than 46 million people worldwide, with a prevalence that is expected to continue to increase in the next 30–40 years.1 AF can have substantial impacts on quality of life and carries an increased risk of ischaemic stroke, heart failure and mortality.2 A recent study in the UK reported a 20% mortality rate within the first year of AF diagnosis, with excess deaths due to AF primarily attributable to cardiovascular disease, infection and metabolic disorders.3 Additionally, AF is economically burdensome to the healthcare system. In the UK, it has been estimated that AF may have resulted in direct healthcare costs of £1,435 million–£2,548 million in 2020, primarily from hospital admissions.4,5
Current European Society of Cardiology (ESC) guidelines recommend a holistic approach to AF management, using the ABC framework to streamline patient care, focused on stroke risk assessment, symptoms and concomitant conditions and risk factors.6 An integrated approach to patient care in AF is aimed at reducing adverse events and mortality through consideration of optimal treatment pathways. Risk factor reduction, medical management with anticoagulation and rate- or rhythm-controlling drugs, and ablation are all potential aspects of an AF treatment paradigm, with careful patient selection being essential when considering the various treatment pathways. With respect to medical management, recent evidence has suggested that early rhythm control through anti-arrhythmic drugs (AADs) or ablation may result in better cardiovascular outcomes than traditional care focused on rate control.7 Overall, AADs are given a higher level of recommendation as first-line AF therapy compared with catheter ablation.6,8 However, recent randomised clinical trials have suggested that endocardial cryoballoon ablation may achieve better clinical outcomes as a first approach compared with AADs in paroxysmal AF.9–11 A meta-analysis of randomised clinical trials also suggested that endocardial radiofrequency (RF) ablation is associated with a reduced rate of AF recurrence, albeit a similar rate of adverse events, compared with AADs.12
Traditionally, if AADs are used as first-line therapy and subsequently fail, ablative therapies are then typically recommended. The appropriate type of ablation in part depends on the type and duration of AF, as well as on the risk factors for AF recurrence and anatomical factors.
Favourable success rates have been achieved with endocardial catheter ablation for drug-refractory paroxysmal AF, which is defined as terminating spontaneously or with intervention within 7 days of onset.13 In paroxysmal AF, endocardial catheter ablation with RF or cryothermal (cryoballoon) energy is primarily focused on isolation of the pulmonary veins (PVs), which were identified in 1998 as being the primary source of AF triggers.14 Pulmonary vein isolation (PVI) is considered the cornerstone of AF treatment. However, persistent AF (continuous AF for more than 7 days and up to 12 months) and longstanding persistent AF (continuous AF for more than 12 months) are more difficult to treat with conventional PVI-focused endocardial ablation (Supplementary Material Figure 1). Better outcomes for these patients are typically reported with surgical ablation; however, it is limited in scope because a more invasive procedure is usually performed concomitantly with planned cardiac surgical procedures. Hybrid AF ablation aims to leverage the advantages of endocardial and surgical ablation and offset their limitations in a standalone, minimally invasive procedure. In this review we examine the evolution of hybrid epicardial–endocardial ablation from the spectrum of percutaneous endocardial and surgical epicardial ablation techniques and provide a perspective on potential advances to hybrid ablation procedures.
Endocardial Ablation
Two recent clinical trials suggested that newer endocardial catheters may achieve favourable results in persistent AF occurring for up to 6–12 months in duration. Freedom from atrial arrhythmias without initiating new or higher dose AADs was 54.8% at 12 months following cryoballoon ablation in patients with a mean persistent AF duration of 7.2 months.15 One repeat ablation was permitted in the 3-month blanking period. In another study, patients with a mean persistent AF duration of 15.9 months had a freedom from atrial arrhythmias off new or higher dose AADs of 61.7% at 15 months with RF catheter ablation (including up to two repeat ablations in the 6-month period following the index procedure).16 Even with these advances, there are some limitations to endocardial ablation. One confounder is the phenomenon of endocardial–epicardial dissociation wherein the endocardial and epicardial surfaces have offset phase and activation patterns.17 In effect, endocardial-only (or epicardial-only) ablation may not be sufficient. Additionally, endocardial ablation focused on PV isolation does not address extra-PV triggers and substrates that become more prevalent in persistent and longstanding persistent AF, such as the left atrial posterior wall and left atrial appendage (LAA).18–21 Although meta-analyses have reported a potential benefit of endocardial posterior wall ablation in PVI, and there is a growing trend to undertake such an approach, there is no absolute consensus on posterior wall ablation that is used routinely.18,22 Measures to reduce thermal injury risk may in turn limit the ability to create durable, transmural lesions. Endocardial ablation focused on PVI has particularly poor results in longstanding persistent AF, with variable results reported for adjunctive substrate ablation.23,24
Cox Maze Surgical Ablation
Concomitant Surgical Ablation
Surgical ablation, notably the Cox maze procedure, is associated with better outcomes in persistent and longstanding persistent AF. The Cox maze procedure was originally a cut-and-sew technique, using a series of incisions and sewing, to create a maze of transmural conduction blocks to prevent macro re-entry circuits. The first Cox maze procedure was performed in 1987, and subsequently 32 patients received the original Cox maze I procedure.25,26 The technique was then modified due to the observation that some patients could not generate an appropriate sinus tachycardia during exercise and had occasional left atrial dysfunction. Incisions around the sinoatrial node were eliminated and replaced with a right atrial counterincision, with additional modification to move the left atrial dome incision posteriorly. This became the Cox maze II, which was performed in 15 patients.26 However, the changes in the Cox maze II technique required complex surgery to the superior vena cava (SVC), including pericardial patching of one incision into the SVC orifice, and did not completely resolve issues with Cox maze I. Interatrial conduction was found to be delayed using both techniques. Thus, the Cox maze III lesion pattern was created, in which again the left atrial dome lesion was moved posteriorly to the extent that it was posterior to the SVC, enabling easier exposure of the left atrium. The evolution from Cox maze I to Cox maze III both protected the sinoatrial node and improved interatrial conduction compared with the previous iterations. In the first 123 patients treated with these cut-and-sew techniques, three early deaths occurred and the most common complications were fluid accumulation (resolved with spironolactone) and perioperative atrial arrhythmias. At a single centre, long-term rhythm outcomes of the cut-and-sew Cox maze procedure in 198 patients were highly favourable, with an estimated 92% freedom from AF at 14 years in patients who had Cox maze as a standalone procedure, and 97% freedom from AF in patients who had a concomitant procedure at 10 years.27 However, overall, the cut-and-sew technique was not widely adopted due to its technical difficulty and substantial morbidity, primarily left atrial dysfunction and pacemaker implantation.
Linear ablation using various energy sources was evaluated. Ablation with RF and cryothermal energy devices offered an alternative means of creating transmural lesions in order to block abnormal conduction as an alternative to cutting and sewing. Gaynor et al. reported the first prospective outcomes of the Cox maze IV procedure, which replaced most of the incisions with RF and cryothermal ablations, leaving only one left atrial and two right atrial incisions.28 Early results suggested a high level of clinical success, with 6-month freedom from AF of 91% with no operative mortality. Outcomes of the cut-and-sew maze technique have been compared with those of Cox maze IV ablation with RF energy augmented by cryoablation, and were found to have similar long-term rhythm outcomes, mortality rates and need for pacemaker intervention.29 The distinction is that the use of surgical ablation devices substantially decreased the technical complexity and length of the procedure, permitting more widespread adoption. Experimental data in explanted human hearts have demonstrated that two applications of bipolar RF energy can achieve 100% transmurality.30
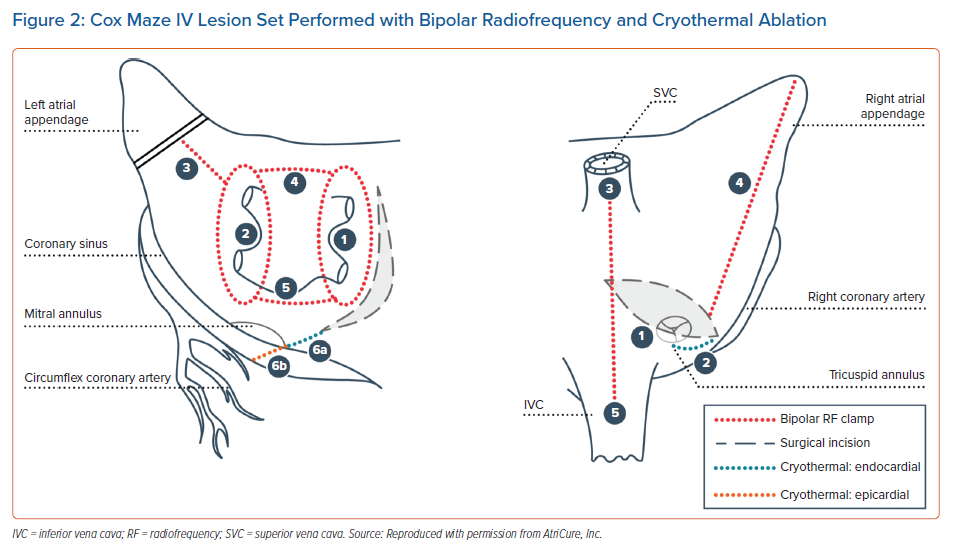
Since the initial report, an investigational device exemption clinical trial and post-approval study reported the safety and efficacy of Cox maze IV procedures using bipolar RF (Figure 1) and cryoablation, and long-term success with surgical ablation concomitant with cardiac surgery procedures.31–34 An important consideration to reported outcomes after surgical ablation is the lesion set used. A true Cox maze IV lesion set is biatrial (Figure 2). Left atrial lesions are focused on right and left PV antrum isolation with roof and floor connecting lines, a connecting lesion to the LAA, and the mitral valve annulus.31 Right atrial lesions include the SVC and inferior vena cava, right atrial appendage lines to the freewall and tricuspid valve annulus, and tricuspid valve annulus to atriotomy. The LAA is also closed. To complete the Cox maze IV lesion set, RF ablation is used for most lesions, with cryoablation applied to the tricuspid and mitral valves to avoid valvular stenosis. Surgical ablation can also be performed with cryoablation only, however, the Cox maze III lesion set is performed in this case. Observational studies have compared cryothermal surgical ablation with RF ablation alone and RF with adjunctive cryoablation. Vural et al. reported that there was no significant difference in sinus rhythm restoration at 12 months between surgical ablation performed with cryothermal energy only compared with RF energy.35 The authors created identical lesion sets with cryothermal and RF energy, however, the lesions shown are not the full Cox maze lesion set and do not show a coronary sinus lesion. Additionally, a limitation of RF-only ablation is that the mitral and tricuspid annular pathways cannot be fully addressed. As previously mentioned, cryothermal energy is preferred because it preserves the collagen matrix and avoids valvular stenosis. Ad et al. compared the outcomes of concomitant Cox maze III with cryothermal energy with those of Cox maze IV with RF and adjunctive cryothermal energy.36 Sinus rhythm restoration regardless of AADs was statistically similar between the cryothermal and RF–cryothermal groups. However, off AADs, sinus rhythm restoration was higher with cryothermal ablation at 6, 36 and 60 months after the procedure, with fewer cardioversions and catheter ablations during 60-month follow-up. Embolic stroke rate was significantly decreased with cryothermal-only ablation.36
Based on its efficacy without the addition of increased operative mortality or morbidity, concomitant surgical ablation with mitral valve surgeries has been given a class 1A recommendation and a class 1B recommendation in non-mitral valve surgeries by the Society of Thoracic Surgeons (STS).37 The ESC/European Association of Cardio-Thoracic Surgery (EACTS) guidelines assign a class 2a (level A) recommendation to concomitant surgical ablation with cardiac surgery.6
Despite the reported positive long-term outcomes and consensus recommendations, concomitant Cox maze IV procedures are still not performed across the board in patients with preoperative AF who are undergoing planned cardiac surgery. In an analysis of the STS database between 2011 and 2014, 48% of patients with AF who underwent non-emergency cardiac operations had concomitant AF ablation.37 After propensity matching, patients who had surgical ablation had improved 30-day mortality and stroke rates. Lower rates of concomitant surgical ablation have been reported with non-mitral valve surgeries, despite published evidence suggesting that they have similar safety and effectiveness to concomitant ablation with mitral valve surgeries.38,39
Standalone Surgical Ablation
Not all patients who have AF are candidates for or are in need of a primary cardiac surgery procedure, such as coronary artery bypass grafting or valve repair. Long-term outcomes of standalone Cox maze procedures performed via sternotomy or right thoracotomy have been reported.40,41 Procedures were performed with cardiopulmonary bypass support. Ad et al. analysed the STS database from 2012 to 2016 and found that standalone AF ablation procedures increased by 7% during that time period.41 However, based on the database, most standalone surgical ablation procedures between 2014 and 2017 were performed off-pump. Off-pump surgical ablation is performed on the beating heart and therefore atriotomies and endocardial lesions are not made.
Thoracoscopic Ablation
Video-assisted and totally thoracoscopic approaches to surgical ablation have been described.42,43 Epicardial RF ablation is restricted to the left atrium and is used to isolate the PVs and make other lesions such as roof line, floor line and ganglionated plexi, and the LAA is closed. The totally thoracoscopic maze (TT maze) procedure uses bilateral port access to isolate the PVs, create a box lesion and trigonum line, and close the LAA.44 The Wolf mini-maze procedure includes video-assisted thoracoscopic PV isolation, ganglionated plexi ablation and LAA closure.45 The two procedural terms are sometimes used interchangeably, however, one difference in the approaches is that the mini-maze procedure uses 5–6 cm bilateral incisions for working ports whereas the TT maze procedure uses smaller bilateral working ports.45 Tricuspid and mitral valve annulus lesions that are usually made with cryoablation in the Cox maze IV procedure are not created with these epicardial approaches.
The effectiveness of epicardial surgical video-assisted thoracoscopic ablation has been shown to be superior to that of endocardial catheter ablation in a randomised trial, albeit with higher procedural complications.46 However, success rates reported in the literature are generally higher with full, on-pump Cox maze IV surgical ablation than epicardial surgical ablation (93% versus 80% with anti-arrhythmic agents, respectively, and 87% versus 72% without anti-arrhythmic agents, respectively).47 Potential reasons for this are that epicardial-only lesions are restricted to the left atrium, and it is difficult to ensure transmural ablation when ablating only from the epicardial surface.
Hybrid Epicardial–Endocardial Ablation Strategies
The concept of a hybrid epicardial–endocardial ablation procedure was developed in part to combine advantageous aspects of existing surgical and electrophysiology approaches into a minimally invasive technique that could be performed on the beating heart by a multidisciplinary team. In particular, key criteria to be met were the creation of transmural, contiguous lesions through both epicardial and endocardial ablation, the use of intraoperative, anatomical lesion visualisation (by the surgeon) and subsequent electro-topographical mapping (by the electrophysiologist) to verify procedural success, and the promotion of multidisciplinary patient care.48 There are two main approaches to hybrid ablation, distinguished by the means of pericardial access and epicardial ablation technique. One hybrid ablation approach accesses the posterior left atrium thoracoscopically and the other approach achieves left atrial access endoscopically through a subxiphoid (or, less commonly, transdiaphragmatic) incision. Overall, the goals of each approach are similar: to overcome the limitations of epicardial-only surgical ablation and endocardial-only catheter ablation by combining techniques to isolate the posterior left atrium and PVs.
Hybrid Thoracoscopic Ablation
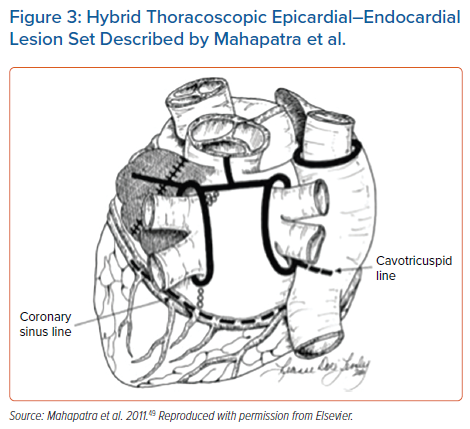
In 2011 a thoracoscopic hybrid epicardial–endocardial technique was described by Mahapatra et al. (Figure 3).49 This procedure differs by approach, which is totally thoracoscopic, and ablation requires different RF devices. The epicardial lesion set is aimed at isolating the posterior wall and PVs, with endocardial ablation used to address gaps identified by electrophysiological mapping. The LAA can be addressed thoracoscopically as concomitant with the ablation procedure. A meta-analysis of hybrid thoracoscopic ablation compared with endocardial catheter ablation found higher freedom from atrial arrhythmia recurrence with the hybrid technique, although complications were increased with hybrid ablation.50 The authors noted that six of the 13 studies on hybrid ablation included early procedural experience or complications in some of the first patients treated. Seventy-one per cent of patients were in sinus rhythm off AADs at least 12 months after the hybrid procedure, compared with 50% with catheter ablation.50 Individual complications that were higher with hybrid thoracoscopic ablation included bleeding requiring transfusion, conversion to sternotomy, cardiac tamponade, hospital mortality, pacemaker implantation, phrenic nerve injury, pneumothorax, and insignificant PV stenosis. Rates of other complications such as bleeding requiring reoperation, groin haematoma requiring therapy, PV stenosis requiring stenting, and stroke/transient ischaemic attack were statistically similar between the groups. In general, there are some safety considerations for unilateral thoracoscopic hybrid ablation with respect to the potential for prolonged unilateral lung ventilation, duration of hospital stay, and occurrence of common complications such as pleuropericarditis.51
Preliminary outcomes from a randomised trial comparing thoracoscopic hybrid ablation with catheter ablation were recently reported.52 In 41 patients, 83% of patients who received hybrid ablation were free from atrial arrhythmias without AADs at 12 months compared with 45% who received endocardial catheter ablation (p=0.015), with a similar quality of life and no reported increase in major adverse events in the hybrid group. An additional randomised IDE (investigational device exemption) trial and a single-arm trial are pending.
Hybrid Convergent Ablation
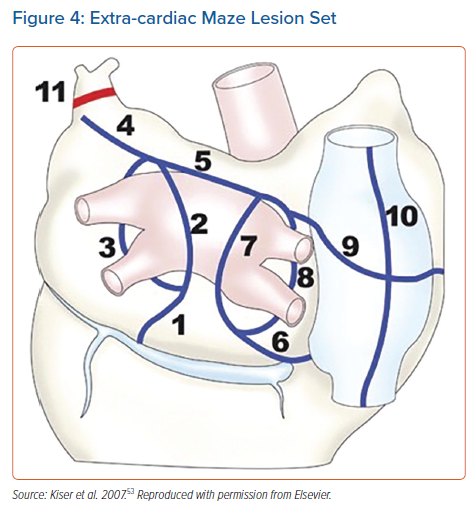
Kiser et al. first described the hybrid convergent procedure, in which epicardial lesions are created by the cardiothoracic surgeon under endoscopic visualisation, followed by electroanatomical mapping and catheter ablation by the electrophysiologist to complete PVI and address gaps left by the epicardial lesion set.48 Unlike traditional epicardial surgical ablation using bipolar clamps, a unipolar, irrigated RF catheter was used through a pericardioscopic cannula with a guidewire. In this respect, the hybrid convergent procedure is the least invasive hybrid procedure currently available. As with thoracoscopic hybrid ablation, pre-existing pericardial adhesions may be prohibitive for the use of the hybrid convergent procedure in patients who have had prior cardiac surgery. The initial epicardial lesion set was extensive and closely resembled the extra-cardiac maze lesion set, which was originally performed concomitant with cardiac surgery as described by Kiser et al. in 2007 (Figure 4), and later as a paracardioscopic minimally invasive procedure.53,54 This included linear ablation of the posterior PV antrum, as well as ablation on the anterior aspect of the PVs, along the coronary sinus, ligament of Marshall, and SVC. Following surgical closure, electrophysiological mapping was performed to guide endocardial catheter ablation, which was focused on PVI, coronary sinus isolation and cavotricuspid isthmus (CTI).48 The goal of the original combined procedure was to achieve isolation of the posterior left atrium and PVs, isolation or conduction block of the coronary sinus, and CTI conduction block.
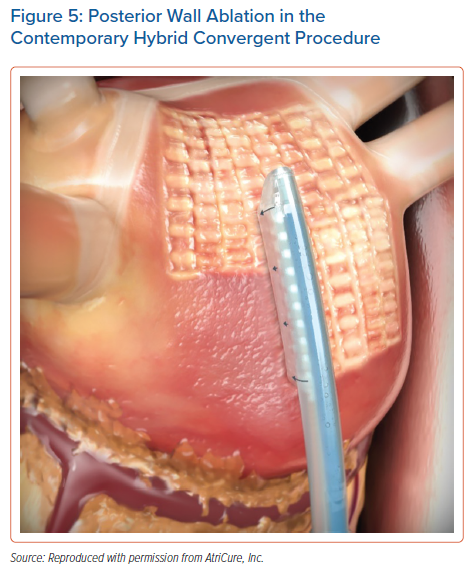
Several studies were published that used this early lesion set and early generations of the unipolar RF device.55–57 Overall, the rhythm outcomes achieved were favourable for a population of primarily persistent and longstanding persistent AF patients, with only one study reporting unfavourable outcomes.57 Early experience with the procedure indicated some safety concerns, including a few reports of atrio-oesophageal fistula.55,57 In part this risk was mitigated through oesophageal temperature monitoring and saline irrigation of the pericardial space. The lesion set also evolved over time, which may have also helped avoid collateral damage. As illustrated in a recent review by Wats et al., epicardial lesions that required manipulation and curving of the distal end of the catheter were eliminated, and epicardial ablation was focused exclusively on the left atrial posterior wall.58 Posterior wall isolation through a box lesion set was originally reported as having additive benefit to surgical ablation clinical outcomes.59 The left atrial posterior wall has a propensity to harbour AF triggers and substrate in persistent and longstanding persistent AF. A substantial posterior wall reconnection rate has been reported following endocardial catheter ablation only, therefore a hybrid epicardial–endocardial ablation strategy may be better to support durable posterior wall isolation.20 Several studies reported the use of an epicardial posterior wall box with hybrid convergent ablation.60–62 Then, the CONVERGE trial introduced the contemporary epicardial lesion pattern, which consists of posterior wall homogenisation through the creation of parallel, overlapping rows of contiguous lesions using the most recent generation of unipolar RF catheter (EPi-Sense, AtriCure, Inc.) in a linear configuration (Figure 5).63 During the time of the trial the primary access technique to reach the left atrium also changed, from dividing the central tendon of the diaphragm to using a subxiphoid incision for cannula insertion (Supplementary Material Figure 2). This change avoided the potential for postoperative development of transdiaphragmatic hernias. Two studies have reported significantly fewer complications in cases in which the subxiphoid approach was used compared with the transdiaphragmatic approach.64,65 Wats et al. published a comprehensive review of clinical outcomes following hybrid convergent ablation that spanned this evolution of the procedure, from its early inception to the lead-up to the CONVERGE clinical trial.58
The CONVERGE trial randomised patients with symptomatic, drug-refractory, persistent and longstanding persistent AF to hybrid epicardial–endocardial convergent ablation (PVI, posterior wall isolation and CTI) or endocardial RF catheter ablation (PVI, roof line, CTI and complex fractionated atrial electrograms at the discretion of the operator).66 Patients in the trial had a mean AF duration of 4.4 years. The trial met its primary effectiveness endpoint, demonstrating significantly higher freedom from atrial arrhythmias off new or a higher dose of AADs of 67.7% through 12 months compared with 50.0% for catheter ablation. It also met the primary safety endpoint, with a 7.8% major adverse event rate within 30 days, below the pre-specified safety goal. Several recent observational studies have reported clinical performance and safety results using the same lesion strategy and unipolar device as CONVERGE, including one propensity score-matched study in which the lesion strategy and device were compared with endocardial catheter ablation.64,65,67,68 A recent review by DeLurgio et al. discussed the results of CONVERGE with regard to the published observational studies that compared hybrid convergent ablation with endocardial catheter ablation, the observed reduction in AF burden that has been reported during follow-up after the procedure, and outcomes in the long-standing persistent AF population.69
As hybrid convergent ablation evolved, physicians performing the procedure gained experience to develop best practices with respect to institutional set up, multidisciplinary heart team collaboration, patient selection, medication strategies, procedural execution and follow-up care.70 Overall, there is recognition that the set up of the procedure does not consist simply of a sequential performance of the surgical and electrophysiological ablation procedures, but instead requires extensive planning and collaboration often coordinated by the electrophysiology team.70,71
Future Perspectives for Hybrid Convergent Ablation
Some outstanding questions remain for hybrid convergent procedures that could be the focus of future studies. The epicardial and endocardial portions of the procedure can be performed on the same day of hospitalisation or alternatively can be staged separately by several weeks, which can be influenced by institutional logistics or reimbursement for healthcare providers. Outcomes for both strategies have been reported but not statistically compared in a case-controlled study. The role that prior catheter ablation plays in the outcome of hybrid ablation is also important for further investigation. CONVERGE included only ablation-naïve patients, but several observational studies have included patients who have received prior ablation. A recent single-centre study compared the recurrence of atrial arrhythmias and AF between patients who had a history of prior ablation before hybrid ablation and those who received de novo hybrid ablation and found no significant differences in these outcomes.72 However, patients who had a history of prior catheter ablation less frequently needed endocardial PVI and cardioversion (to restore sinus rhythm) during the hybrid convergent procedure. Larger, multicentre analyses may be warranted.
CONVERGE and other published studies used irrigated RF catheters for endocardial ablation, however, some investigators have incorporated the use of endocardial cryoballoon in hybrid convergent procedures.65,72 Endocardial cryoballoon was reported to be non-inferior to RF ablation for paroxysmal AF treatment, and the STOP PERSISTENT AF trial demonstrated safety and effectiveness of endocardial cryoballoon for the treatment of early (duration less than 6 months) persistent AF.73,15 Outcomes of hybrid convergent ablation using endocardial cryoballoon have not been formally compared with endocardial RF ablation and may be relevant for future evaluation.
The LAA is routinely closed in Cox maze surgical ablation procedures and can also be managed thoracoscopically. The LAA itself harbours electrical activity and is also the predominant site of thrombus formation in AF.21 Some investigators have reported preliminary data on the addition of thoracoscopic LAA exclusion as part of hybrid convergent procedures, and this is an emerging area for further study.74–76
Conclusion
AF is burdensome to both patients and the healthcare system, with an increasing prevalence worldwide. The genesis of AF is complex and still not entirely clear. AF can be present prior to the observation of structural heart disease, wherein it can contribute to atrial pathologies, such as mitral and tricuspid valve regurgitation. Conversely, the presence and progression of structural heart disease can also create the milieu for AF to initiate and progress through pathological substrate. In most cases, AF is present in the absence of structural heart disease, potentially due to poorly controlled risk factors, comorbidities and/or genetics. In effect, the specific role for and extent of ablation depend on the context of the patient’s clinical condition.
Studies have identified the predominant sites harbouring arrhythmogenic triggers, which include first and foremost the PVs, and then the left atrial posterior wall and LAA, among others. In addition, as AF progresses, triggers outside the PVs become more prevalent, which is a consideration when choosing the ablation strategy appropriate for the patient. The LAAOS III trial demonstrated that closing the LAA in patients with AF undergoing cardiac surgery reduced the rate of stroke compared with leaving the LAA open.77 Therefore, closing the LAA, when accessible, is also a consideration during surgical ablation for AF. It has been nearly 25 years since the first cut-and-sew Cox maze procedure. Since then, the field of AF treatment has seen numerous innovations and procedural advancements with respect to cardiac ablation in both cardiothoracic surgery and electrophysiology. Hybrid convergent ablation is a newer approach that combines aspects of both disciplines to achieve PV and posterior wall isolation. Procedures from across the spectrum, from biatrial Cox maze to hybrid epicardial–endocardial ablation to endocardial catheter ablation alone, have been developed to address the primary sites of AF triggers and substrates across a range of clinical scenarios, taking into consideration the relative invasiveness of the ablation procedure and the minimal ablation targets, to effectively and safely restore normal sinus rhythm (Supplementary Material Figure 3). Careful patient selection when evaluating the available approaches for AF treatment is critical in the context of multidisciplinary, holistic AF care.
Click here to view Supplementary Material.
Clinical Perspective
- Outcomes from endocardial ablation therapy for persistent AF remain suboptimal compared with the treatment for paroxysmal AF, particularly in patients with the long-standing form and those with significantly dilated atria.
- Hybrid ablation is a well-established form of therapy for persistent AF and has better outcomes than endocardial ablation alone, given that the lesions sets are more reliably transmural. However, compared with endocardial ablations, the complication rates from traditional (thoracoscopic) hybrid ablations are greater, owing to multi-port thoracoscopic access to the pericardial space in order to deliver the epicardial ablations.
- Convergent hybrid ablation therapy is relatively new and is the least invasive of all of the hybrid approaches; a single port of entry through a small subxiphoid incision provides direct access to the pericardial space, which circumvents the more traumatic and prolonged transthoracic approach.