AF is an increasingly common condition worldwide, with over one million people affected in the UK alone.1 AF is important due to its associated comorbidities, including risk of stroke and hospitalisations from heart failure.2 Therefore, AF has a considerable impact on both patients and healthcare providers. The treatment of AF is much more than just establishing the need for long-term anticoagulation; research has progressively shown that early rhythm control can improve patient outcomes and that this may be prognostically relevant.3 In addition to older studies on the effectiveness of catheter ablation, there is increasing evidence demonstrating that, as a first-line treatment, it is more effective at achieving rhythm control than medical therapy, without significantly adding to the risk of adverse events.4–10 In line with this evidence, the European Society of Cardiology 2020 AF guidelines recommended that “AF catheter ablation for [pulmonary vein isolation (PVI)] should/may be considered as first-line rhythm control therapy to improve symptoms in selected patients with symptomatic paroxysmal AF episodes and symptomatic persistent AF (without major risk factors for AF recurrence) as an alternative to [antiarrhythmic drug (AAD)] class I or III, considering patient choice, benefit, and risk.”11
Although there is an evidence-based shift to support catheter ablation as early treatment for AF in some international guidelines, changes in real-world practice are often slower due to decisions regarding restructuring of healthcare resource allocation, which cannot be achieved without health economics research to help identify the most appropriate solution. Another challenge is awareness of the updated treatment guidelines by clinicians involved earlier in the AF treatment pathway, including general practitioners and general cardiologists.
In this review article, the importance of health economic data on the treatment of AF is discussed with the aim of creating awareness about the latest evidence and recommendations that will help inform changes in real-world practice.
Definitions
Most economic evaluations in healthcare are cost-effectiveness studies. The unit of cost-effectiveness is measured in cost per quality-adjusted life years (QALYs) gained, where QALY is a general unit to express 1 year of good health (i.e. time and quality of life benefit). Cost utility analysis guides procurement decisions and involves calculation of incremental costs and effects of a certain treatment, combining the unit measure as the incremental cost-effectiveness ratio (ICER; i.e. cost per QALY). Modelling can use data derived from randomised controlled trials or from a combination of data pulled from health and economic data sources, which then go through a decision–analytical process on probability. To manage potential bias, sensitivity analysis can explore potential sources and uncertainty. Probabilistic sensitivity analysis is preferred to assess any uncertainty or unknowns in the model because it allows potentially multiple aspects of uncertainty to be reflected at the same time for a better overall picture. In addition, this type of analysis provides the best estimate for Markov models with multiple non-linear inputs. Markov models are useful in health economics and for a condition such as AF because they evaluate risk over time and have the capacity to allow events to occur more than once. There are assumptions that a patient is in one of a set number of defined health states in the model.
Economic Model Data and Clinicians
Clinicians are familiar with interpreting data relating to the clinical effectiveness of treatments, but understandably less familiar identifying and interpreting economic data. However, economic evaluation of treatment options has always been an important factor in decision making in healthcare systems. For the UK, the National Institute for Health and Care Excellence (NICE) uses clinical and cost-effectiveness data to determine whether certain treatment options should be recommended. In turn, the National Health Service (NHS) uses NICE guidance to inform service or treatment availability and their associated costs. NICE assesses cost-effectiveness to maximise health gain from available resources; this acknowledges that budgets are limited, and bridging scientific data with economic detail can bring best possible care into effect.12 Finding the most appropriate methodology for cost-effectiveness analysis should consider the nature of the condition in question. It is important for a common condition like AF, but the same analysis cannot be applied for much rarer diseases, such as spinal muscular atrophy.13 Since the 1970s, demand for all sectors of health care has increased rapidly and has been met by increasing growth of technology and pharmacological therapy. However, there has not been the same degree of growth in resources to match the demand. Kernick’s review on health economics for the medical physician sums up the importance of economic evaluation and the different types of studies used to match the appropriate scenario.14 Cost-effectiveness is the most common type of study used to compare interventions or treatments for the same condition for similar clinical outcomes. Results are presented in the form of a ratio (e.g. ICER). This may be supported by a cost utility analysis, which offers insights into the quality and quantity of life, with the unit of measure being the QALY.
Cost-effectiveness of Catheter Ablation Versus Medical Therapy
Previous studies on the cost-effectiveness of catheter ablation versus medical therapy used trial data to provide clinical inputs into the model, with short time horizons and a narrow focus on the outcomes measured. This provided the incentive to conduct a new evaluation that may be more applicable to real-world patients. A 2014 study by Reynolds et al. compared the cost-effectiveness of cryoballoon ablation with that of AADs in the UK, finding an ICER of £21,957, which was above the £20,000 willingness to pay (WTP) threshold.15 That study only looked at a time horizon of 5 years and did not consider events such as hospitalisations from heart failure, which may explain the higher ICER for cryoballoon ablation treatment. Hospitalisations or the utilisation of healthcare facilities are important variables to consider in a long-term condition like AF. In addition, the study considered the costs relating to older-generation cryoballoon catheters at that time, which may affect components in the overall costs to the healthcare provider. The improvements in methodology and operator effectiveness within the past decade should have seen improved outcomes, measured by overall freedom from AF, shorter inpatient stays and fewer complications (i.e. reduced healthcare provider costs), which, overall, would make catheter ablation more cost-effective (lower ICER).
A 2019 study performed in Australia by Gao and Moodie looked at the cost-effectiveness of catheter ablation versus medical therapy in patients with both AF and heart failure, yielding an ICER that was above the WTP threshold.16 However, that study only evaluated the impact of reduced mortality. Therefore, again, healthcare facility use and other clinical events were not accounted for, which are important variables with significant effects on overall cost and quality of life.
Aronsson et al. published a cost-effectiveness substudy in 2014 of their randomised controlled trial on first-line treatment of paroxysmal AF using radiofrequency compared with AADs.17 In their substudy of the same population, Aronsson et al. found that ablation was more cost-effective for younger patients (age <50 versus >50 years), but their analysis was concentrated on order of treatment as opposed to defining what treatment should be offered based on age. That study was interesting because the ICER calculated by the authors was different to the cost-effectiveness analysis from a separate study, namely RAAFT, with that the ablation procedure was significantly more expensive in the study by Aronsson et al.18 These clinical randomised studies offered invaluable insights into ablation treatment compared with medical therapy but, when it comes to cost-analysis based on the same data, there are limitations on local cost analysis and its application towards long-term benefit.
In summary, these main studies offer some insights into the economics of ablation with some variation in study design and focus. More of these studies are required to follow on from clinical trials to allow research findings to be interpreted within this context and whether that enhances the study findings. However, it is important to recognise that this approach is still imperfect, because these short- to medium-term results are used to provide economic evaluation over a longer period of time or even lifetime horizon analysis. One way to improve on these analyses is to incorporate real-world data whenever possible.
Use of Real-world Evidence Data
Clinical trials often have protocols that influence usage, are performed at top-performing, high-volume clinical sites and generally have relatively small sample sizes. By using large real-world populations to derive many of the estimates used in a health economics model, the results become more generalisable and can capture benefits that may not be seen in smaller, randomised trials. This approach may become more widespread since NICE announced more routine use of real-world data as part of their 5-year strategy launched in April 2021.19
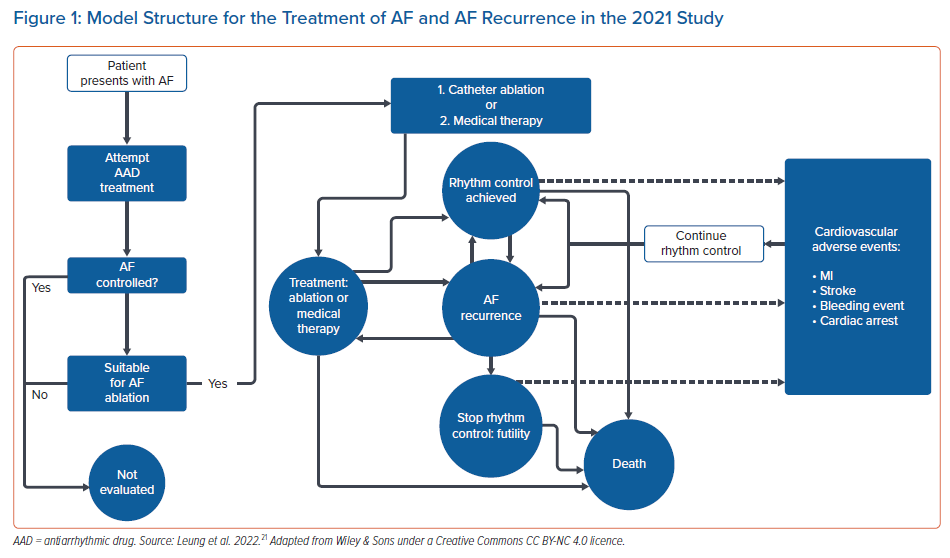
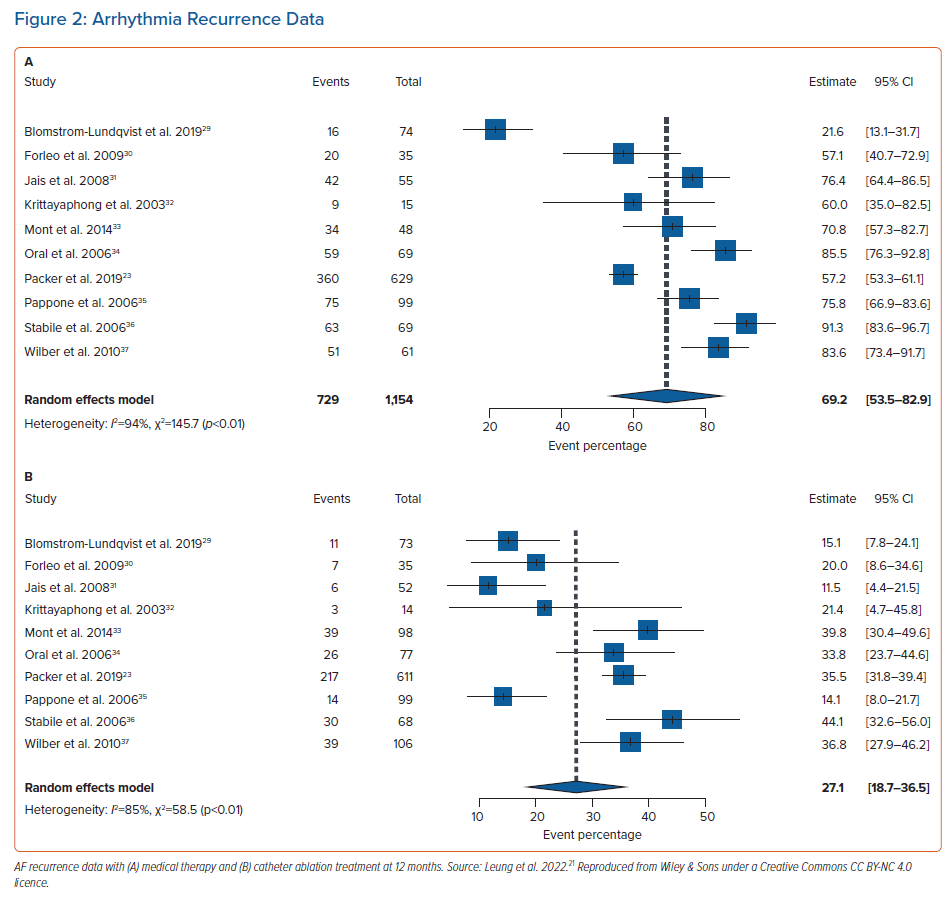
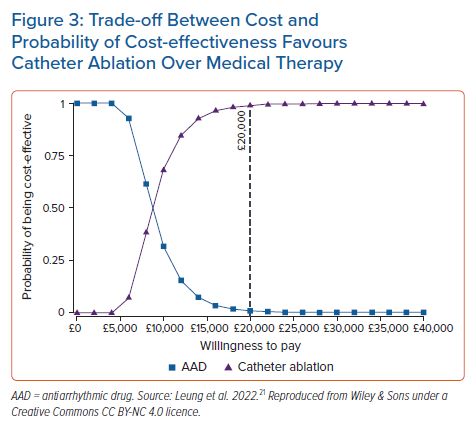
We proposed an economic model analysis of ablation versus medical therapy from the perspective of the UK NHS, with the data summary presented at the European Heart Rhythm Association 2021 and full results now widely available.20,21 In this study, data were extracted from real-world studies and supplemented the systematic literature review and meta-analysis from established clinical trials. Compared with existing model data, the uniqueness of this study rested with the real-world data input. A patient-level Markov health-state transition model was used to conduct a cost–utility analysis. The population included patients previously treated for AF with medical therapy, including those with heart failure, simulated over a lifetime horizon. Figure 1 illustrates AF treatment in the model. Patients entered the model from the age of 64 years. Data sources included published literature on healthcare resource utilisation and cardiovascular event rates in real-world patients, a systematic literature review and meta-analysis of randomised controlled trials for AF recurrence (Figure 2A,B) and publicly available government data/reports on costs relevant to the NHS. From this unique perspective, catheter ablation (by any modality) resulted in a favourable ICER per additional QALY gained compared with medical therapy. Figure 3 illustrates that catheter ablation is a cost-effective treatment at various WTP thresholds, including £20,000. Rhythm control was significantly more successful in the catheter ablation group and sustained this superiority over the years that the patients remained in the model. The cost-effectiveness was evident in time horizons tested beyond the 5-year time constraint of older studies. This study concluded that catheter ablation is a cost-effective second-line treatment for AF compared with medical therapy from the perspective of the UK NHS.20,21
As with other studies, our study has limitations. Although the cost input into the model included state-of-the-art radiofrequency catheters, the clinical evidence evaluated the effectiveness of ablation procedures using older technology. Therefore, the clinical benefit is underestimated. In addition, real-world data were primarily extracted from a US-based population study by Noseworthy et al., which included a large well-matched patient population.22 Although the clinical endpoints should not vary dramatically, there may be differences in patient assessment and clinical practice that would not be translatable to care in the UK. In addition, this model did not account for inevitable crossover from medical therapy to catheter ablation, which is common in clinical practice (e.g. 27.5% of patients crossed over from medical therapy to ablation in the CABANA study), but this was done to ensure a clear comparison of the ablation and medical therapy treatment strategies to assess cost-effectiveness.23 It is important to note that in the current environment, worsened by the coronavirus pandemic, it is not unusual to find patients deemed suitable for catheter ablation remaining on a waiting list for the procedure over a prolonged period, over three cycles (9 months) duration as per the model. In this period, AAD therapy may be used as a bridging measure. This period is akin to a treatment crossover despite original intentions by both patient and specialist opinion, and only adds further to healthcare provider costs, in addition to reducing patient quality of life.
Finally, this model only considers direct costs to the health providers: the NHS and prescribed specialised services. The model does not capture expenses for patients, nor does it consider burdens such as missed time from work, reduced productivity or the burden on caregivers, particularly for those suffering a disabling adverse event.
Recent NICE Guidelines
The latest NICE clinical guideline on the diagnosis and management of AF was published in April 2021.19 This was an update on the 2014 guideline, using recently published clinical and health economic data on the treatment options for AF.
The cost-effectiveness analysis of catheter ablation treatment as second-line treatment (after failure of at least one AAD) found that radiofrequency point-by-point ablation was more cost-effective over a lifetime than cryoballoon or laser balloon ablation.19 The NICE network meta-analysis calculated a 1% difference in AF recurrence between cryoballoon ablation (32%) and radiofrequency point-by-point ablation (31%); however, radiofrequency point-by-point ablation remained the most cost-effective treatment option after scenario analyses adjusting for cost and healthcare resource use.19 This demonstrates the importance of considering 12-month outcomes as part of routine clinical decision making: even a 1% difference in AF recurrence has an impact due to the costs of additional redo procedures. Redo procedures may also add burden to waiting lists, which could limit access to ablation for new patients. Due to the difference in cost-effectiveness, the updated guidelines recommend that patients should be offered radiofrequency point-by-point ablation unless there are specific clinical reasons why an alternative should be used instead (e.g. patient factors or wishes).19 The analysis itself was based on patients with paroxysmal AF and this recommendation was for patients with paroxysmal AF and applied to those with symptomatic persistent AF who cannot have or are unsuitable for long-term AAD therapy.
The NICE analysis used data from randomised controlled trials and health economic studies to populate usage parameters over a lifetime period and it was calculated that radiofrequency using a point-by-point method was cost effective with an ICER well below the current WTP threshold (£9,764 with a WTP threshold of £20,000).
Studies published from 2003 onwards were included by NICE; therefore, some technology may no longer be used. For example, of the 16 studies reporting AF recurrence for radiofrequency point-by-point ablation, only four used contact force (CF)-sensing catheters.24–27 We know from a previous meta-analysis of CF-sensing catheters versus conventional catheters that CF-sensing catheters are more effective at treating AF, with lower rates of AF recurrence at the 12-month follow up.28 And so, although the analysis did not find a significant difference in AF recurrence rates across all modalities, we can extrapolate from this that, with CF-sensing catheters only, radiofrequency may show a superior edge in efficacy.
Applicability of Economic Models
Despite economic models relying on some data inputs specific to the authors’ healthcare systems, the analysis can still be highly useful for the international community as a rough guide: often the clinical and population data are applicable, but there may be differences in regional costs and healthcare resource use. The UK model used clinical data already established in the medical literature and included patients outside of the UK. To our knowledge, it is the first comprehensive economic evaluation of ablation treatment compared with medical therapy that uses real-world data. It is also novel to use cost data for state-of-the-art radiofrequency ablation technology. Thus, the most recent analysis should also be meaningful to international clinicians and healthcare providers.
The latest economic evaluation analysis and the older analyses imply that there should be prioritisation of arrhythmia services where AF ablation treatment is available and an extension of these services to improve accessibility to the population in need by ensuring patients are referred to specialists quickly if their first treatment fails. Regular review of the clinical and economic data is necessary to keep up with incremental changes in technology, first-line approaches and in different AF subtypes. For all clinicians, the updated data should make us question our local and wider arrhythmia service provision and how it meets the current data and guidelines.
AF Subtypes and First-line Treatment
There is public interest and debate over the potential benefits and cost-effectiveness of catheter ablation versus medical therapy, particularly in patients with persistent AF. The recent UK cost-effectiveness study examined patients with all types of AF and did not specifically break out or model patients with paroxysmal versus persistent AF, using population-level treatment effects that were applicable to all AF subtypes.21 This was done for two reasons. First, by evaluating all AF patients, it gives a more comprehensive view of the real-world cost-effectiveness of ablation to inform policy and reimbursement decisions. Second, there is a lack of direct published evidence, particularly in real-world studies, comparing catheter ablation to medical therapy in persistent AF. As more evidence becomes available, it will be important to conduct future health economic research on the subtypes of AF. Although results on AF were not split into subtype in this study, a subgroup of those with heart failure and AF were analysed and, in fact, ablation treatment was most cost-effective in this scenario, which matches the known clinical benefit of ablation for those with persistent AF and related cardiomyopathy.21
The same limitations apply to questions surrounding the health and economic benefits associated with first-line treatment of AF; as clinicians adopt the European Society of Cardiology recommendations on second-line catheter ablation treatment, this should generate real-world data to inform future analyses.
Conclusion
Economic model analyses have shown that catheter ablation is a highly cost-effective treatment for AF compared with medical therapy from the perspective of the UK. Alongside increasing evidence of the clinical effectiveness of catheter ablation, AF ablation services should be prioritised with clear referral pathways to make ablation more accessible to the population in need.
Further Work
Economic modelling has informed and influenced the latest NICE guidelines on the management of AF and, in general, this data analysis has the power to change healthcare resource allocation. Further work is recommended to improve the use of these data to guide future guidelines and treatment recommendations: real-world data inputs into economic models can help create a more accurate analysis of treatment costs for the same clinical condition, and therefore a more effective use of resources. In addition, previously published randomised trials focused on ablation treatment as a second-line treatment but, with the emerging data from recent randomised clinical trials investigating the effectiveness of ablation versus medical therapy as first-line treatment, economic model analysis in this context would provide further supportive insights into the benefits of early rhythm control and how this can be put into effect within the constraints of our healthcare systems.
Clinical Perspective
- Economic evaluation is essential to bridge advances in scientific evidence towards implementation of updated best-practice guidelines and improve healthcare services to the public.
- Economic evaluation can be improved by the utilisation of real-world data in addition to clinical trial data inputs because real-world data are more reflective of the current clinical situation and therefore more applicable in real-world care.
- For the treatment of AF, further work on economic evaluation is required, particularly to look at AF subsets separately and the use of ablation as first-line treatment.