Of late, there has been a significant resurgence of interest in the writings of Ivan Mahaim. We have ourselves recently produced a series of reviews in which we discussed the features of the unusual arrhythmias that are now usually explained on the basis of anatomical substrates bearing his name.1–3 It has recently been suggested, however, that the eponym ‘Mahaim’ should be discontinued.4 We have sympathy for this viewpoint. We accept that anatomical substrates of arrhythmias are better described in descriptive fashion, not least because one of us reported the first clinical case of a ‘Mahaim fibre’ in 1971, while another of us was involved in an attempt to establish the concept of descriptive nomenclature as long ago as 1976.5,6 We are also strong supporters of the need for descriptive nomenclature to be attitudinally correct.7 We wonder, however, whether discontinuation of the eponym, as suggested by those writing recently in Heart Rhythm, will produce the suggested clarification of the underlying substrates for the abnormal rhythms?4
Despite the very best efforts of those who have sought to avoid eponyms in general, it is unlikely that the name will disappear. It is also the case that a detailed reading of the writings of Mahaim reveals much to be admired.8–10 Mahaim himself cannot be blamed for the misinterpretations of these writings by subsequent generations of electrophysiologists. In this review, in defence of Mahaim, we summarise the initial findings of this Belgian, who practised throughout his career in Switzerland, discussing their relevance to modern-day electrophysiologists. It will then remain to be seen whether the eponym disappears from the electrophysiological lexicon.
The Career of Ivan Mahaim
Mahaim was born in Liege, Belgium, on 25 June 1897.11 He was educated in Switzerland, where he entered the University of Lausanne in 1918 to study medicine, receiving his degree in 1925. Having undertaken postgraduate training with Wenckebach in Vienna, and LeClerc in Paris, he returned to Lausanne in 1929, where he was appointed associate professor at the university. During his career, he published 100 manuscripts, mostly in his native French, but with some significant contributions in the English literature. His magnum opus was published early in his career, and was entitled Les Maladies Organiques du Faisceau de His-Tawara. In this book, he described detailed findings of his study of over 400 individuals with problems of rhythm originating from the atrioventricular conduction axis.
Many of Mahaim’s investigations were supplemented by equally detailed histological studies. As we will describe, he revisited these early experiences later in his career, when summarising his findings in the descriptions of the pathways that earned him the eponym. As we will also discuss, and as was pointed out by Lüderitz when summarising his overall achievements, he showed great prescience.11 Lüderitz points to his speculations with regard to the potential of cardiac surgery.
Mahaim was also remarkably prescient in contemplating the role of the alternative structures that are now interpreted in his name as the substrates for pre-excitation, even though the concept on which he based his interpretations proved to be spurious.11 He was also very much a ‘renaissance man’. In the latter part of his life, he established a reputation as a world-class scholar in the achievements of Ludwig van Beethoven.11 It is, nonetheless, his significant achievements in describing the detailed anatomy of the atrioventricular conduction axis, and its direct connections with the ventricular septal myocardium, on which we will concentrate.
The ‘Paraspecific’ Myocardial Connections
Mahaim began his research into the diseases that might afflict the myocardial connection between the atrial and ventricular myocardial masses at a time when the substrate for atrioventricular conduction remained controversial. This was because, although Wilhelm His Junior had provided an excellent account of the bundle that now bears his name, the English physiologist, Arthur Stanley Kent, had continued to maintain in the second decade of the 20th century that multiple myocardial pathways crossed the insulating planes of the atrioventricular junctions.12,13 In making these claims regarding multiple myocardial connections across the atrioventricular junctions in the normal heart, Kent was unequivocally wrong. He was partially correct in so far as the structures he illustrated do, indeed, exist. We now know that they are remnants of a ring of histologically specialised tissue that, in the developing heart, surrounds the embryonic interventricular communication. With ongoing normal development, the ring becomes remodelled as the direct connection is established between the cavities of the right atrium and right ventricle. The specialised myocardial tissues normally become sequestrated within the vestibule of the tricuspid valve. They persist as the entities discovered and illustrated by Kent.
As is recognised in the title of Mahaim’s book, it was Tawara who clarified the arrangement of the solitary pathway that, in the normal heart, provides the substrate for atrioventricular conduction. This is the pathway described by Mahaim as being ‘specific’ for atrioventricular conduction. On the basis of his research, specifically on the interpretation of the electrocardiographic changes, he inferred that other connections must exist between the atrial and ventricular myocardial masses to permit conduction when the major pathway was itself diseased. It was these additional pathways that he named the ‘paraspecific’ connections. He summarised his findings in this regard in the review published in 1947.9 In this work, he speculated that the pathways may not be confined to the connections that he had previously observed extending between the major pathway itself, but could also involve the structures illustrated by Kent. Mahaim, therefore, was well aware that pathways other than those we now name in his memory could provide alternative substrates for atrioventricular conduction.
The Pathways Described by Mahaim
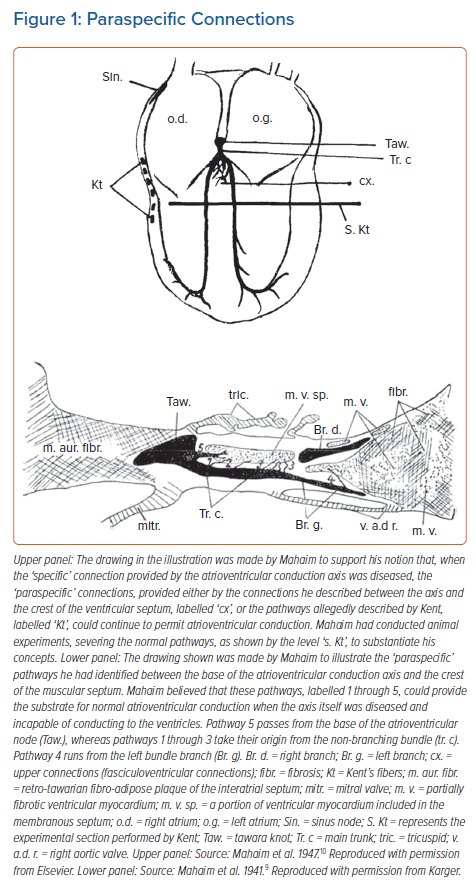
It was direct connections between the base of the atrioventricular conduction axis and the crest of the ventricular septum that had been the focus of Mahaim’s histological studies. In his animal experiments, he had made cuts, as shown in the upper panel of Figure 1, observing that atrioventricular conduction persisted despite the severing of the mid-septal components of the ventricular bundle branches. These findings, along with the interpretations of the electrocardiograms obtained from patients known to have acquired diseases of the conduction axis, underscored his proposal that the histological connections he identified (Figure 1, lower panel) could provide the alternative pathways necessary to provide the observed atrioventricular conduction.
We recently had the opportunity to assess the findings of Mahaim through our own access to a series of data sets prepared so as to provide serial sections through a series of atrioventricular conduction axes obtained from adults.14 Our initial assessment of these data sets was confined to examination of the atrioventricular node and the penetrating atrioventricular bundle. Within this analysis, we did not encounter any nodoventricular connections, although we did find one connection between the origin of the penetrating bundle and the crest of the ventricular septum. Such a pathway was shown by Mahaim as #5 in the illustration we have now reproduced (Figure 1, lower panel).
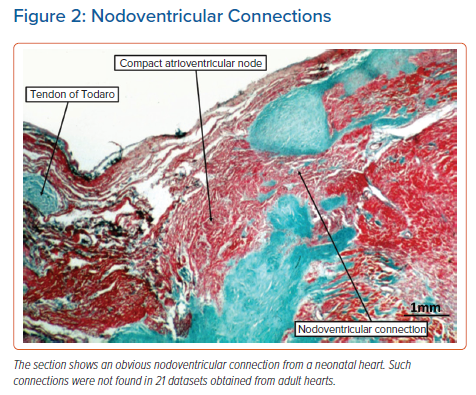
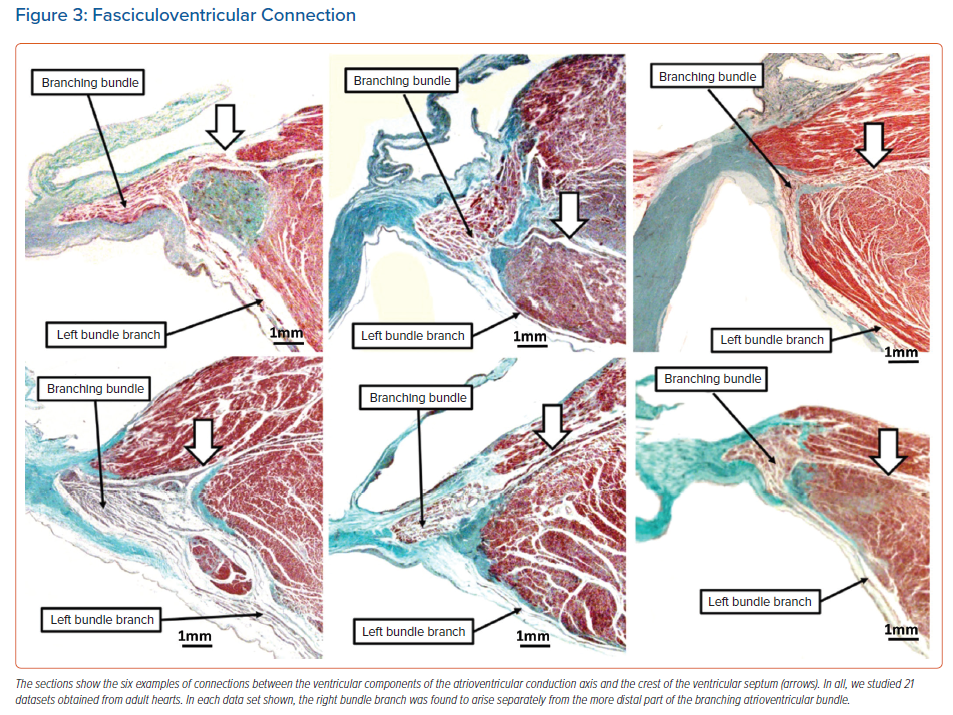
In our previous analysis of hearts obtained from neonates and infants, it had been usual to find extensive remnants of the atrioventricular node dispersed within the insulating fibrous tissues of the atrioventricular junctions.15 These arrangements have previously been described as ‘foetal dispersion’. They were implicated by some investigators as a potential anatomical substrate for sudden infant death, although that hypothesis was never proven.16 In some neonatal hearts, the remnants can be sufficiently extensive to produce obvious nodoventricular connections (Figure 2). We were unable to identify such connections in our adult data sets.14 We have now extended our analysis of these data sets to include the non-branching and branching components of the conduction axis. This has permitted us to identify with certainty the origin of the right bundle branch. We were surprised, therefore, to find, in addition to the pathway described at the origin of the penetrating atrioventricular bundle, several further examples of fasciculoventricular connections (Figure 3).
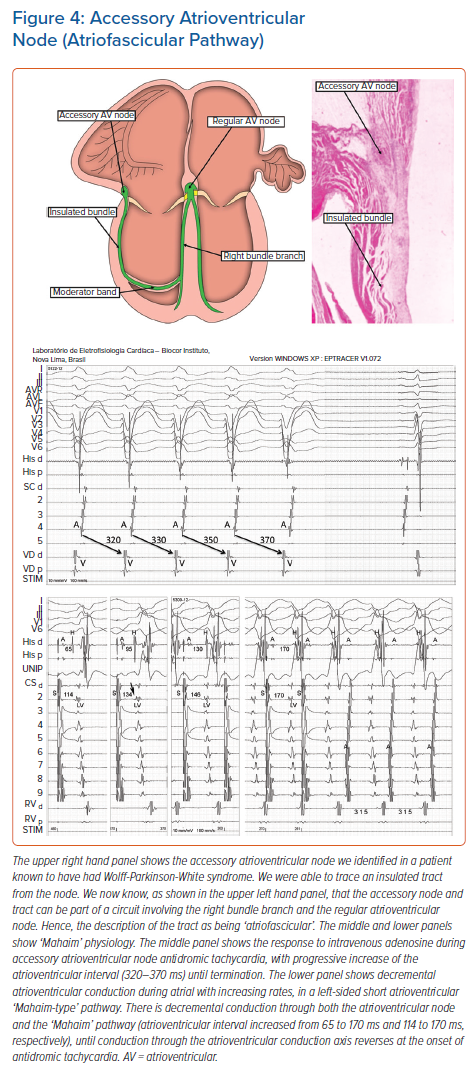
As is well-known to electrophysiologists, and now responsible for much of the confusion regarding the use of ‘Mahaim’ as an eponym, the arrhythmias described in his name can also be produced by a quite different anatomical substrate. This is the so-called ‘atriofascicular pathway’. The use of this term is not without its own problems. When the morphological members of the European Study Group on Pre-excitation suggested names for the anatomical substrates producing pre-excitation, they proposed that Mahaim’s ‘paraspecific’ pathways should be subcategorised as either nodoventricular or fasciculoventricular connections.6 This suggestion has been well received. At the same time, the study group proposed that the so-called ‘atrio-Hisian’ connections should be dubbed atriofascicular pathways. Nowadays, of course, the pathways considered to be ‘atriofascicular’ are far removed from the para-Hisian area. These pathways, nonetheless, can produce electrophysiological findings remarkably similar to those believed to produce ‘Mahaim’ pre-excitation through the paraspecific pathways. The substrate now believed to produce the alternative ‘Mahaim pre-excitation’ was first identified by ourselves, although we were unaware, at the time, of the significance of our finding. In our analysis of hearts obtained from patients dying with known pre-excitation, we discovered an accessory insulated atrioventricular pathway in a patient with Ebstein’s malformation, but also known to have regular Wolff-Parkinson-White syndrome (Figure 4, upper right panel).17
This patient was also shown to have well-formed, left-sided, muscular atrioventricular connections. The right-sided connection we found was unusual, as it took its origin from an accessory atrioventricular node of the type initially illustrated by Kent. We were able to trace an insulated tract arising from the node, but we could not identify the ventricular insertion of the tract. It was the experience of Klein et al. that revealed how, in certain circumstances, the tract could pass into the moderator band and unite with the right bundle branch.18 It was this experience that prompted description of the pathways as being ‘atriofascicular’. The findings validate the existence of additional ‘paraspecific’ pathways involving the structures illustrated by Kent, thus pointing again to the prescience of Ivan Mahaim.
As we now know that the so-called atrio-Hisian pathways almost certainly represent the final connection between the atrial myocardium and the atrioventricular conduction axis prior to its insulation as the His bundle, there is no longer a need to employ ‘atriofascicular’, as initially suggested by the European Study Group.14 The way is clear, therefore, to stratify the anatomical substrates for so-called ‘Mahaim pre-excitation’ using the descriptive terms of nodoventricular, fasciculoventricular and atriofascicular pathways. However, does this mean we then need to strip Mahaim of his eponym?
The Electrophysiological Significance of Mahaim Arrhythmias
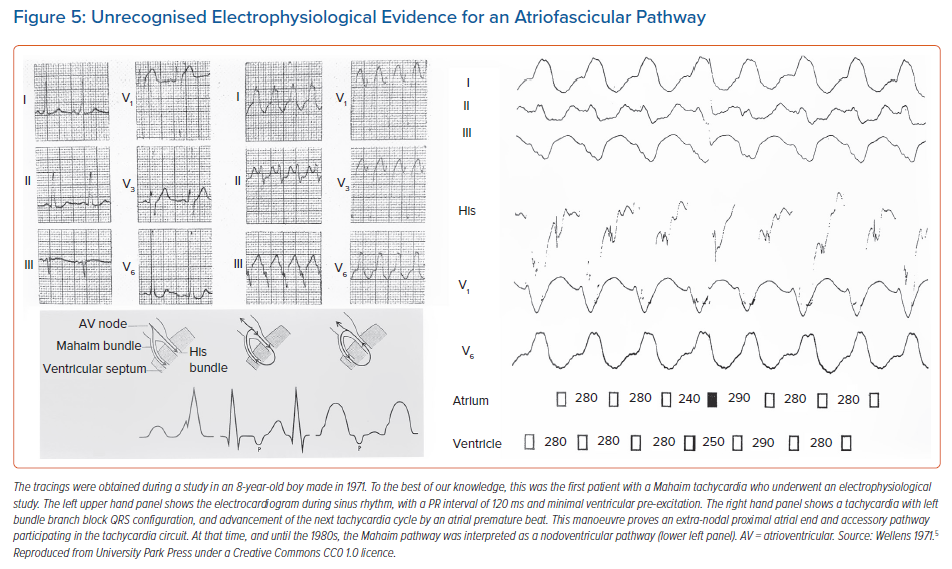
It is certainly true that all accessory myocardial pathways having slow and decremental properties of conduction, regardless of their specific morphology, share a common electrophysiological characteristic. It is this property that has widely become known as Mahaim physiology (Figure 4, middle and lower panels). In keeping with this approach, the different variants of pre-excitation produced by the pathways are now commonly lumped together as ‘Mahaim fibres’. Already in 1971, one of us had published a case of a young boy suffering from tachycardias.5 In Figure 5, we show details from this first patient with Mahaim tachycardia who underwent an electrophysiological study. At that time, only the presence of an anatomical nodoventricular connection, as described by Mahaim, could explain the unusual findings. In retrospect, the findings of the gradual decremental increase in ventricular pre-excitation, the development of a QRS configuration with the features of left bundle branch block during atrial single test stimulation, and the reproducible initiation and termination of the tachycardia by appropriately timed atrial and ventricular premature beats, could also have been produced by an accessory atrioventricular pathway having nodal-like properties. This, of course, is the substrate described by Klein et al. 17 years later, and currently known as the atriofascicular pathway.18
Among the connections identified by Mahaim himself, it was only the fasciculoventricular variety that had not been implicated in re-entrant circuits.19 Recently, however, cases have been reported involving a circus movement tachycardia, supposedly incorporating a fasciculoventricular connection.20 The fasciculoventricular pathways, more usually, function as bystander conduits in other tachycardias, such as atrioventricular re-entry or atrioventricular nodal re-entrant tachycardia. We know that anterograde conduction through such bystander pathways needs to be identified correctly during an electrophysiologic study, so as to avoid harm to the atrioventricular node should the fasciculoventricular pathway be mistakenly targeted for ablation. Fasciculoventricular pathways seem to be more frequent than atrioventricular bypass tracts, and probably ubiquitous structures.21 Thus, in our ongoing studies, we found fasciculoventricular pathways in 14 of 21 data sets prepared from normal hearts.22
In the other variants of ventricular pre-excitation involving a ‘Mahaim’ pathway, macro-reentry in the circuits can lead to antidromic tachycardia.23 This is because the circuits involve anterograde conduction over the pathway with decremental properties, and retrograde conduction through the normal atrioventricular conduction axis. The retrograde conduction may occur via the right bundle branch or through the branches of the left bundle. These variations can change the ventricular-His timing, as well as the tachycardia cycle length, giving rise to complex tachycardias.24,25 The atriofascicular pathway involving an accessory atrioventricular node, and usually located at the acute margin of the right atrioventricular junction, is the most frequent structure involved. The macro-reentrant tachycardia using such a circuit would better be described as an antidromic accessory atrioventricular nodal re-entrant tachycardia.
Short decrementally conducting accessory pathways originating from the base of the compact atrioventricular node, which may occur at both the right and the left atrioventricular junctions, could produce comparable circuits.2 These nodoventricular pathways can be used anterogradely in a circus movement tachycardia, with ventriculo-atrial dissociation, as the atrial myocardium is not part of the circuit. They can also be used retrogradely, giving rise to narrow complex tachycardias with atrioventricular dissociation.3 These pathways, which are those described by Mahaim, can also be involved in other uncommon arrhythmic mechanisms, such as spontaneous automatic rhythms, or non-paroxysmal tachycardia due to double atrioventricular conduction. More recently, they have been implicated in nodoventricular-atrial re-entry, which may cause pseudo-atrioventricular block.26–28
Conclusion
As parts of groups, we have been involved for several decades in this long process of unravelling the historical anatomical and electrophysiological characteristics of the variants of ventricular pre-excitation. We endorse the notion that cardiac structures are best described according not only to their structure-function relations, but also according to their correct attitudinal position inside the thorax of the living person.1 We also take the stance, nonetheless, that some eponyms, providing they have withstood the test of time for the medical community at large, need not be discarded simply for the sake of the ‘political correctness’. Terms, such as His, Purkinje and Wolff-Parkinson-White, are not only well-known, but are part of our past. The same goes for the name of Ivan Mahaim. These eponyms are an integral part of the heritage that defines us. We are unaware of any evidence showing that use of the terms, ‘Mahaim pathway’ or ‘Mahaim physiology’, negatively impacts the message to be conveyed as any patient undergoes an electrophysiological study, in which the object is to establish the specific mechanism of the arrhythmia, and the location and structure of its anatomical substrate. Only when this object is achieved can the proper treatment be determined.
It remains the fact that recognition of the potential presence of a so-called ‘Mahaim pathway’ means that there will be fun at the electrophysiology lab! Eponyms have not disappeared, nor are they likely to go away. The main issue with their use is to be sure that the honouree is worthy of the structure thus named. Ivan Mahaim, in our opinion, remains more than worthy of the reference, as long as it is properly used.