Normalisation of heart rhythm in patients vulnerable to atrial arrhythmia is now largely procedural rather than pharmacological.1 It is based on ablation of the myocardium in lines to obstruct arrhythmia circuits, or on destroying or encircling zones of the myocardium that trigger arrhythmia. In AF, ablation necessarily includes isolation of the pulmonary veins; for persistent AF, more extensive lesion sets are often used, particularly isolation of the posterior wall.2,3 Unlike paroxysmal AF, right atrial lesions are also often required. Lesion sets in persistent AF are often directed at targets, such as sites of automaticity, fractionation or low amplitude that may be found in a variety of locations, including the left atrial appendage, the crista terminalis and the superior vena cava, locations seldom targeted in other conditions.
Transmurality and contiguity are prerequisites for an effective lesion set. For an endocardial delivery of thermal energy to create tissue necrosis to the epicardial surface, some tissue injury must occur beyond that limit. To achieve consistent transmurality across a large lesion set, the operator must accept that damage will extend into adjacent structures. A skilled and experienced operator can do this in a controlled manner, but the risk is not eliminated.
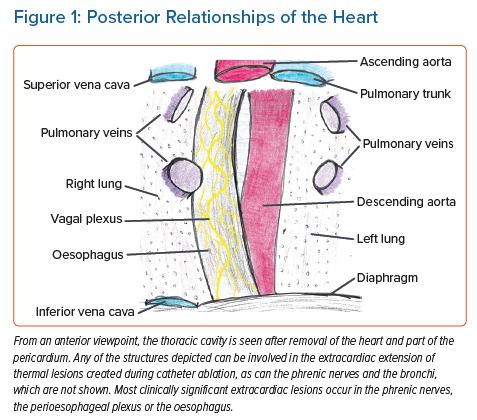
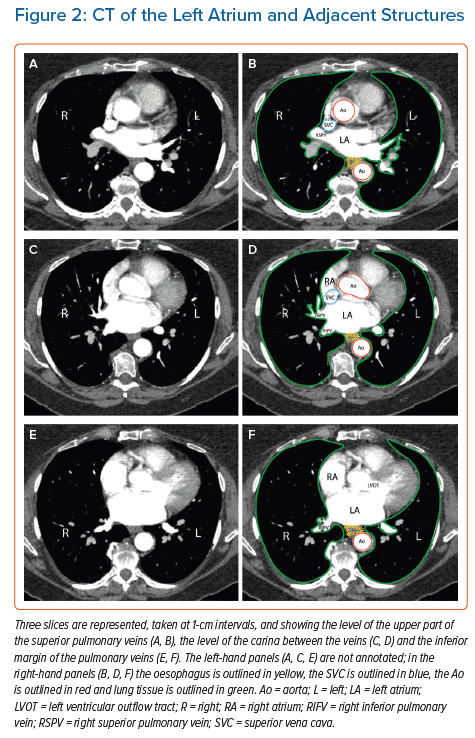
Most of the structures that abut the human atrium (Figure 1) tolerate such injury without threat to life; small amounts of lung tissue and the diaphragmatic muscle can be considered dispensable, although symptomatic injury to the lung and airways does occur in cryoballoon ablation.4 Injury to the oesophagus is different: atrio-oesophageal fistula (AEF), as well as fistulae from the oesophageal lumen to the mediastinum or the pericardial space, have been reported in approximately 0.1% of ablations for atrial arrhythmias and most cases are fatal.5–8 Fistulae from the atrium to a bronchus have also been reported; like AEF, they are often lethal.9 The true incidence of atriobronchial fistulas is not known, but radiological studies have highlighted the close proximity of the lung to the left atrium (Figure 2), in particular along the course where ablation of the pulmonary veins would be performed.10 Nerves are also vulnerable, particularly the perioesophageal neural plexus and the phrenic nerves.
Importance of the Oesophagus
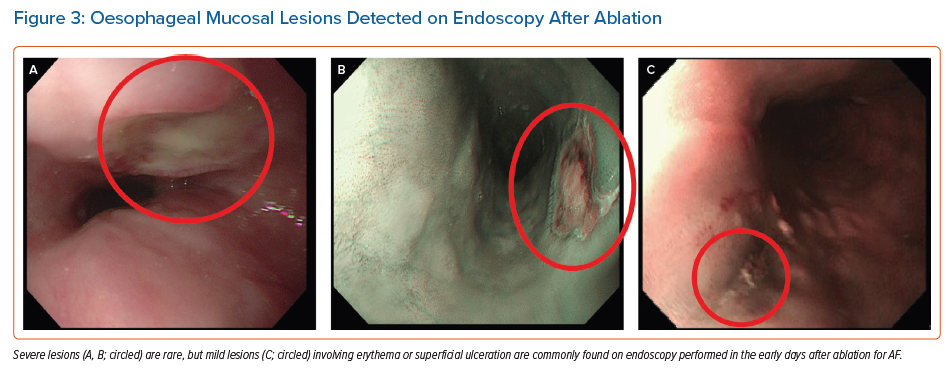
In its infancy, ablation therapy for AF was dogged by a risk of complications related to the practicalities of catheter placement and energy delivery. Vascular injuries at the access site, cardiac injuries, thromboembolism and pulmonary vein stenosis were all common. The incidence of all of these injuries has declined steadily, progress that is attributable, in part, to technical refinements, such as the use of ultrasound to guide vascular puncture. It may also be related to accumulated experience among the community of physicians performing ablation. AEF has been stubbornly resistant to this progress, with an incidence remaining at approximately 0.01–0.3% over the past two decades.5,9 Evidence of milder thermal oesophageal injury (Figure 3) can be found in as many as 47% of cases.11 As other complications decline, and as procedure numbers increase, AEF becomes ever more important.
Perioesophageal Neural Injury
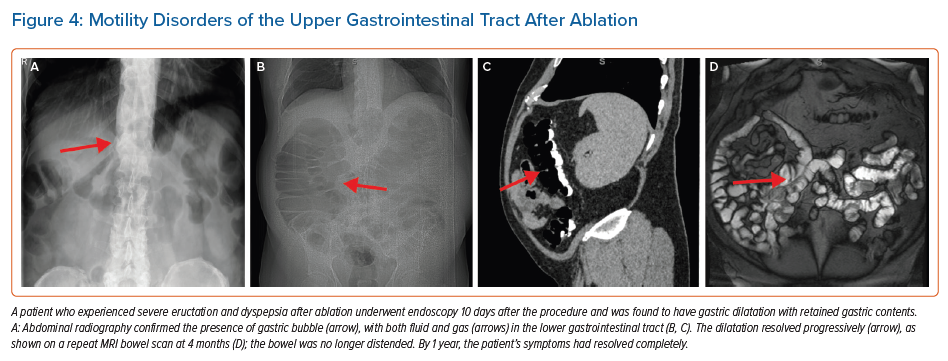
The perioesophageal vagal plexus influences the function of the gastrointestinal tract, including the gallbladder; clinical issues faced by those who experience vagal plexus injury as a result of catheter ablation include gastroparesis, bowel hypomotility (Figure 4) and acalculous cholecystitis.12,13 In a study involving 535 patients, 13 (2.4%) patients experienced severe gastric hypomotility, which, for the majority, gradually resolved over a period of 4 months.14
Yakabe et al. conducted a study of the utility of preprocedural CT to predict acute gastroparesis after AF ablation.15 They found that cases of confirmed gastroparesis (symptom profile, X-ray radiography or other imaging evidence) were more likely to have a ‘middle-positioned’ oesophagus (located in line with the spinal vertebrae) and additional posteriorly directed lesions, suggesting that the factors that promote injury to the plexus parallel those responsible for injury to the oesophagus.15 It may be hoped, although should not be assumed, that the same protective strategies may be applied to both sets of factors.
Other Neural Injury
Autonomic responses, including profound changes in the sinus rate or the occurrence of atrioventricular block, are commonly seen during AF ablation, particularly when delivering therapy around the superior pulmonary veins. The sites where such responses are elicited correspond to the known location of autonomic ganglia, so these events are often termed ‘ganglionic responses’ and are believed to result from damage to the ganglia. This is unique among the extracardiac effects of ablation in being regarded as beneficial: the occurrence of ganglionic responses is correlated with a successful ablation, and no downside to the effect has been reported.16 Evidence suggests that this represents a direct benefit of ganglionic injury, rather than ganglionic responses being just an indicator of lesion transmurality.16
The right phrenic nerve lies close to the right superior pulmonary vein and is vulnerable to ablation at its ostium. This is particularly a feature of cryoballoon ablation, where some degree of transient phrenic nerve dysfunction occurs in as many as 25% of cases, but clinically important injury occurs in fewer than 1% of cases.17
The left phrenic nerve is vulnerable to injury during ablation in the left atrial appendage. Ablation in this area is not a routine part of AF ablation in most centres, so is not seen as a major issue. When it does occur, it can be permanently disabling.
Strategies for Avoidance of Injury
The process of thermal injury progressing to a serious complication could be interrupted by: avoiding the delivery of thermal insult to the myocardium; delivering energy only at sites well removed from vulnerable structures; creating smaller lesions; moving vulnerable structures away from the site of delivery; intervening to control the temperature in the vicinity of vulnerable structures; or interrupting the sequence of events that progresses from initial injury to serious consequences. Some of these strategies can be facilitated by precise localisation of the structure at risk. All these strategies have been explored and all are in routine use in some form.
Avoiding Lesions in Areas of Danger: Anatomical Considerations
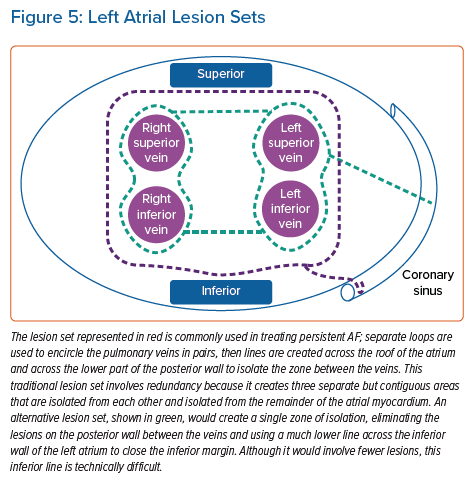
Ablation lesions can extend to approximately 7–9 mm from the tip of a catheter delivering radiofrequency (RF) energy, but slightly less for current cryotherapy catheters.18 The distance from the atrial chamber to the oesophageal lumen is shortest in the posterior wall; it is more than 15 mm at the anterior margin of the pulmonary veins.6,7 In principle, a lesion set composed of the deepest lesions possible could encircle the veins and the posterior wall without ever coming in reach of the oesophagus (Figure 5). In practice, this line is difficult to accomplish: it would involve a line across the roof of the left atrium more anterior than is usual, and a line across the floor that is much more inferior and anterior than usual. The more convenient alternatives that are in common use approach within 5–10 mm of an oesophagus lying in its typical position.7
The position of the oesophagus is neither predictable nor constant. In some cases, it is closer to the right pulmonary veins; in others, it is closer to the left. Even within the course of a single procedure, the oesophagus may move from one side to the other.19 Precise identification of the position of the oesophagus in real time is a logical part of any strategy of avoidance. Because procedures are typically guided by fluoroscopy, it makes sense to place an object or substance in the oesophageal lumen that makes it visible: contrast agents, nasogastric tubes and temperature probes are commonly used. A simple tubular design in the oesophageal marker weakens the strategy because the wall of the oesophagus most at risk of injury may still lie at a distance from that marker. None of these anatomical avoidance strategies has been evaluated in isolation. In real practice, because the risk of AEFs may occur despite a minimum lesion set created for pulmonary vein isolation, the strategy of the operators falls back on limitation of ablation power and time, for any posteriorly directed lesion.
The distance from the atrial cavity to any large airway is longer than the distance to the oesophagus. The left and right main bronchi are visible to the operator on the fluoroscopic imaging used for most ablation procedures. Because of the rarity of serious injury to the bronchi, operators do not usually take specific measures to avoid them.
Establishing the position of the phrenic nerve and minimising ablation in its vicinity is vital when using RF energy in the superior vena cava, in the left atrial appendage or inside the right superior pulmonary vein (as opposed to its antrum). Intraprocedure imaging cannot detect the nerve, so it is localised by stimulating electrically at high amplitude and looking for phrenic contraction. In cryoballoon ablation, phrenic nerve injury is avoided by eschewing deep engagement of the right pulmonary veins, and the severity of injury is minimised by stimulating the nerve proximally throughout the procedure and terminating therapy promptly at the earliest sign of weakening. A similar approach can be used in treating the superior vena cava.
Minimising Lesion Depth Near Sensitive Structures
Lesion depth in RF ablation is a function of the power and duration of delivery and the force of catheter contact with the myocardium. Lesion size and shape are also influenced by features of catheter design, such as the mode of irrigation and the size of the tip electrode, as well as by the orientation of the catheter with respect to the endocardial surface. Confounding factors, such as local blood flow, make it difficult to predict the exact size of any lesion produced by any one delivery of energy, and the accumulation of heat in the tissue further complicates matters when successive lesions are delivered close together and in quick succession.
There is clear evidence that delivering more energy increases the risk of serious oesophageal injury; protocols that are designed to produce shallower lesions have been shown to protect against endoscopically detected oesophageal thermal lesions, including limitation of contact force.20,21 The success of this strategy requires that the depth of the lesion is accurately predicted: to this end, algorithms that incorporate all important determinants are commonly used. Combined with a disciplined consistency in interlesion distance and timing, most variables contributing to lesion depth are controlled.
Moving the Target
Most of the structures that are at risk during ablation are relatively fixed in location, but the oesophagus is mobile with respect to the heart. This movement can be inconvenient for an operator trying to avoid it, but the phenomenon can also be harnessed. A transoesophageal echo probe can be used to move the oesophagus to the left when ablating near the right veins and to the right when ablating near the left veins.22 The range of movement achievable is such that even lesions in the middle of the left atrial posterior wall can be kept at more than 1 cm from the most lateral part of the oesophageal lumen. However, this method cannot be standardised for every patient, and moving the oesophagus often leaves a trailing edge, which gives the false impression of adequate displacement. There are also dedicated devices designed for the purpose of oesophageal deviation in left atrial ablations with varying results from experimental use.23–27 As yet there are no randomised trial data to validate this method of oesophageal protection.
Altering the Ablation Modality
Myocardial ablation is currently most often performed by heating the tissue using RF energy. Cryotherapy is also commonly used, and pulsed field energy is an emerging modality. Ablation using laser or ultrasound energy has been described, but neither is currently in routine use.28 Because most ablations historically have been done with RF energy, and because most reported cases of oesophageal injury are from RF cases, RF ablations serve as a reference against which other modalities can be judged.
RF energy is an ancient technique by the standards of interventional electrophysiology, but it can be applied in new ways. High-power, short-duration ablation was originally applied to deliveries of 40–50 W, typically for a period of 10–20 s; more recently, a power of 90 W for just 4 s has entered routine use.29–31
Pulsed field ablation shows promise as a method for ablating the myocardium without collateral damage to adjacent structures.32,33 Because the injury produced by pulsed field energy is proportional to cell size, the myocardium is disproportionately affected and the tissues of the oesophagus are relatively spared. This promised theoretical advantage awaits rigorous testing in clinical practice. Preliminary results are encouraging: in a series of 121 consecutive cases of ablation for AF performed with pulsed field energy, no instance of clinically significant oesophageal injury was reported.33
Cryotherapy has been linked to cases of life-threatening oesophageal injury. The prevalence of injuries appears to be less than that associated with RF energy, but no randomised comparison has been performed of sufficient size to determine the relative risk.34 Most cryoablation for atrial fibrillation has been done using a single brand of balloon-tipped catheter, but other products are now available. No systematic evaluation of the risk of oesophageal injury has been conducted with any of these products.
Oesophageal Luminal Temperature Monitoring
Clinical studies have shown that an increase in oesophageal luminal temperature is linked to oesophageal injury and so, in standard practice, oesophageal temperature probes are widely used to guide the procedure, particularly during the creation of ablation lesions on the posterior aspect of the left atrium.35–52 Various different models of temperature probe have been used in conjunction with different ablation protocols. This form of oesophageal protection is the most common in routine practice, apart from limitation of ablation power, time and contact force. Despite its long-term use in clinical practice, the method was not evaluated scientifically until recently. Trial evidence is discouraging: the OPERA study, a recent landmark randomised trial, showed no evidence of benefit.35 On balance, the evidence suggests that the use of a temperature probe has the opposite effect to that intended, increasing the risk of oesophageal injury.48,49 This effect could relate to the physical presence of a foreign object in the oesophagus, or just reflect a less cautious approach when operators perceive that they are protected by a device.
Ex vivo studies also highlight the limitations of temperature monitoring; each model of temperature probe has its own physical profile and response time, which differ widely.53 The extent of protection, if any, is not standardised between probes and opens up the question as to the usefulness of this strategy at all when other studies show that use of a probe did not offer additional protection compared with controls.35,36
Controlling the Luminal Temperature
The lumen of the oesophagus can be cooled or warmed by fluid infused into the lumen or by a device placed in the lumen. Direct injection of water has been used in three controlled trials, each individually inconclusive but significant in concert.54 Building on this success, a cooling device that offers thermostat-controlled adjustment of the luminal temperature in the range 4–42°C (EnsoETM; Attune Medical) has been found to offer significant protection in a randomised trial of 120 patients evaluated by endoscopy.55
The EnsoETM device offers features that cannot be achieved by the infusion of saline: first, the protection is controlled in the sense that the temperature is set at a level chosen by the operator and maintained constant by a thermostat; and, second, the device is highly effective because the flow rate through the tube is 2.4 l/min, sufficient to convey a far larger amount of thermal energy into or away from the oesophageal lumen than can be achieved by any other available method. Direct infusion of a cooling fluid via an open-ended catheter is limited by the ability of the body to absorb that fluid. In a 90-min procedure, the EnsoETM circulates >200 l water, more than an order of magnitude greater than can be achieved without recirculation.
Control of the oesophageal temperature has been applied to cryoablation as well as to RF methods, and is undergoing clinical evaluation in an ongoing multicentre randomised trial (NCT04079634). The warming balloon marketed in parallel with the Adagio Ultralow cryoablation system is irrigated, but the extent and physical dimensions have not yet been studied in detail. The whole equipment is therefore quite new compared with other ablation modalities and unknown with regards to comparative safety, efficacy and efficiency parameters.
Secondary Prevention of Collateral Injury
Most injuries to extracardiac structures do not require specific treatment. Phrenic nerve palsy either resolves spontaneously or remains permanently. Injuries to the perioesophageal plexus resulting in gastroparetic symptoms may be confirmed by imaging, but the treatment is conservative.
Again, the oesophagus is different. Injuries progress from local tissue injury through necrosis to fistula formation, a process that may be due to luminal bacteria and acid. The slow progression of this process presents an opportunity for early intervention, but only for the vigilant. The assessment of those who may have sustained oesophageal injury requires knowledge of the condition by the initial medical responder. Often the clinical presentation is with fevers, and blood cultures and blood tests may reveal bacteraemia.56,57 Symptoms may include chest pain, and clinical examination may reveal acute neurological deficits. Any of this array of symptoms after ablation should alert the clinician. Early surgery is usually required.58–60 The placement of oesophageal stents, which involves a less invasive procedure, has a role, at least when fistulation is localised to the pericardium.
Summary of Preventative Strategies
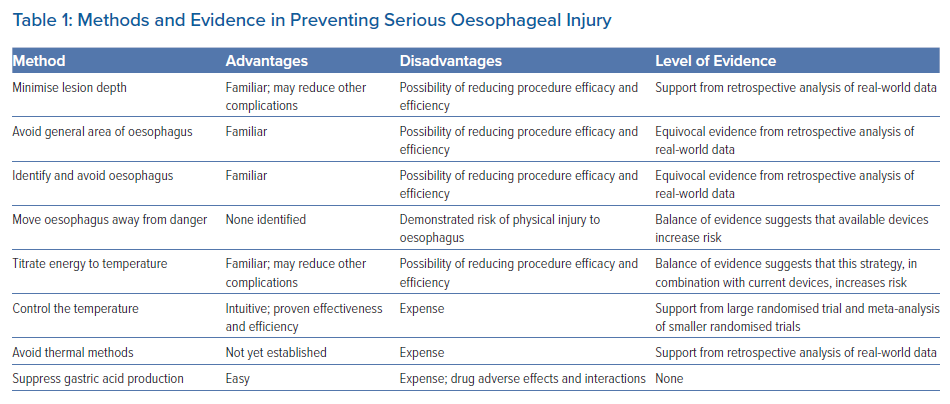
Among the preventative strategies described, most are unsupported by randomised trial data (Table 1). Although it is widely accepted that reductions in contact force and power can reduce significant thermal injury in RF ablation, this and oesophageal temperature monitoring are not backed up by trial data. Active oesophageal temperature control has shown clear benefit in randomised trials, and oesophageal deviation devices are undergoing evaluation.54,55 Oesophageal protection in cryotherapy has received less attention, but is important, especially now that ultra-low cryotherapy has emerged.
No single strategy has emerged to prevent all extracardiac injury. In practice, many strategies are used in every case, and it is appropriate that a mix of strategies should continue to be used. In a matter of patient safety, every plausible protection should be employed.
Conclusion
The extension of ablation-related injury into extracardiac structures is an important cause of complications of AF ablation. Structures at risk include the phrenic nerves and the bronchi, which are routinely protected by minimising lesion creation in their vicinity. Protection of the oesophagus is the highest priority due to the lethality of oesophageal injury. The oesophagus and the nerve plexus surrounding it have traditionally been protected by monitoring of the intraluminal temperature, but the evidence does not support this approach. Oesophageal cooling does provide effective protection during RF ablation. Pulsed field ablation as an alternative to thermal methods offers protection against any extracardiac injury in theory due to its specificity for the myocardium, but real-world data are needed.
Clinical Perspective
- Effective ablation requires transmural lesions, meaning that some injury must extend beyond the heart.
- Injury to the phrenic nerves and the perioesophageal neural plexus is an important complication of ablation for AF.
- Injury to the oesophagus is common but usually mild.
- Severe oesophageal injury is an important cause of ablation-related death.