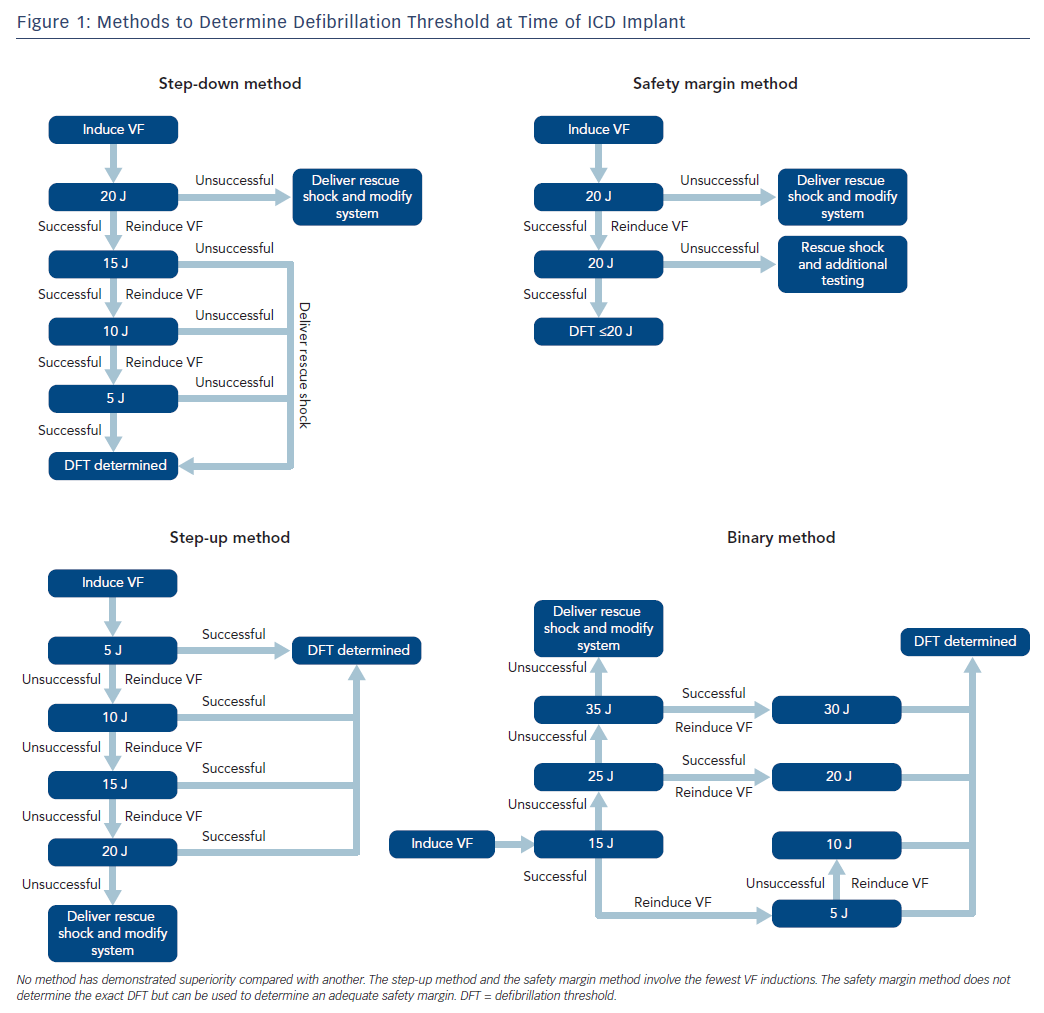
The ICD is an important therapy for both primary and secondary prevention of sudden cardiac death in selected patients. The role for defibrillation threshold (DFT) testing either intraoperatively or postoperatively has changed significantly over the past few decades, and it is no longer routinely recommended in patients undergoing left-sided transvenous ICD implantation.1–7 The definition of the DFT is a probabilistic value and is historically defined as the minimum energy required at which two shocks can successfully terminate VF, which dates back to the era of surgically implanted devices with epicardial patches.8 Such testing was routine for all ICDs in the past due to uncertainty surrounding the device and a desire to predict probability of success in treating ventricular arrhythmias. Generally, devices are programmed with a safety margin of at least 10 J, although some trial data indicates that a 5 J margin could provide equal efficacy.9 A number of different methods can be used for determining DFT, and no particular protocol has been shown to be superior compared with others (Figure 1). Additionally, some operators have employed the use of upper limit of vulnerability, which correlates well with the DFT and has been used in place of direct DFT testing with good reliability.10,11
While routine DFT testing in left-sided transvenous implants is no longer recommended, it is currently a class I recommendation to perform DFT testing for subcutaneous ICDs (S-ICD) although evidence to support this is limited.7 This review will address the considerations for DFT testing with transvenous ICDs including risks and contraindications, examine the impact of DFT testing on patient outcomes and explore some of the data for DFT testing in S-ICDs. We will also discuss our current approach to DFT testing during device implantation.
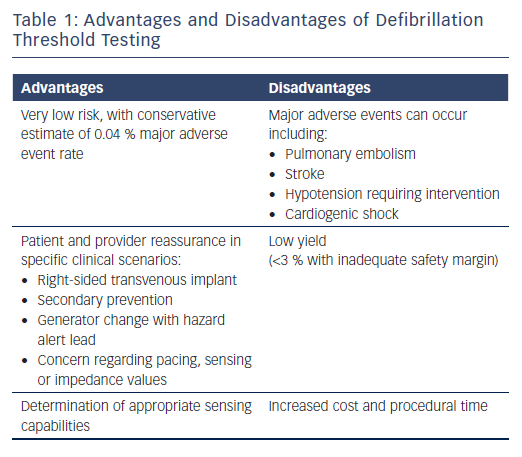
Managing Inadequate Safety Margins and Risks of Defibrillation Threshold Testing
The ‘yield’ of DFT testing reveals an inadequate safety margin, leading to system revision. Observational data of DFT testing in the modern era has demonstrated a diminishing yield to ≤3 % as devices and techniques have improved.6 High output active generators with biphasic waveform and programmable waveform tilt have significantly reduced the incidence of inadequate safety margins.12–15
Although uncommon, when inadequate safety margins are encountered, various techniques can be employed to achieve a lower DFT. The single coil lead has become more commonly used, owing to data suggesting similar efficacy with fewer long-term lead complications compared with dual coil leads.16 However, dual coil systems may achieve a lower DFT and can be considered in the case of an inadequate safety margin.17
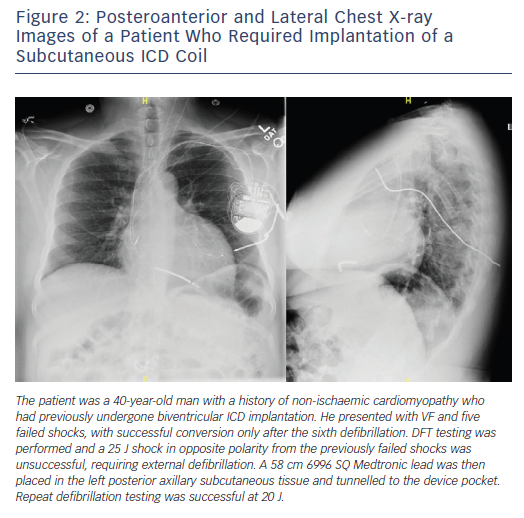
In the Inhibition of Unnecessary RV Pacing With AV Search Hysteresis in ICDs (INTRINSIC RV) study, 59 of 1,530 patients tested required system revision due to an inadequate safety margin.18 These revisions included reversing polarity, repositioning the RV lead, adding a subcutaneous array, or repeating testing at a later date after medical optimisation. In a study by Vischer et al., nine of 436 patients (2 %) tested required system revision by modifying the superior vena cava coil to either on or off, repositioning the lead and/or generator, adding a subcutaneous array, or adding a coronary sinus coil.19 Another study by Guenther et al. revealed 11 of 783 patients (1.4 %) tested required revision to achieve adequate DFT.3 These changes included reversing polarity, lead revision or adding a subcutaneous array. Another strategy, reported by Cesario et al., involves the implantation of an azygous vein coil.20 Specialised equipment for certain modifications is not standard, but can be acquired from device manufacturers (Figure 2).
Although the risk of DFT testing is low, these risks must be carefully weighed against its potential benefits. Large registry data suggest the risk of major complications including stroke, pulmonary embolism, cardiogenic shock, or hypotension requiring resuscitation is an estimated 0.17–0.4 % and the risk of mortality is 0.016–0.07 %.21,22 The benefits of DFT testing include the ability to identify inadequate sensing of VF or an insufficient safety margin, which can prompt system revision to appropriately treat sudden cardiac death (Table 1). Kolb et al. performed a risk–benefit analysis, using figures of a reduction in mortality of 7–8 % using ICDs and an assumed yield for DFT testing of 2.5 % (likely to be an overestimate, considering that the study was published in 2009 and there have been improvements to newer devices).23 They found that the mortality prevention rate with DFT testing is less than 0.2 %, corresponding to one potential death being averted for every 500 tests.23 This suggests that methods of risk stratifying for those most likely to benefit from DFT testing are needed.
Selecting Patients for Defibrillation Threshold Testing
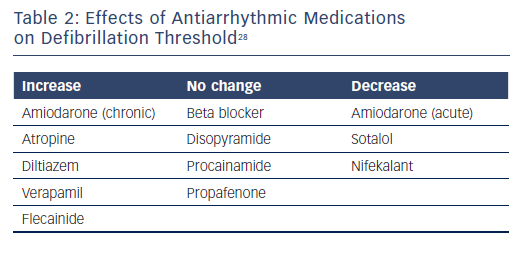
Observational and registry data have identified certain risk factors as predictive of a higher DFT or an inadequate safety margin. Traditional risk factors include patients with non-ischaemic cardiomyopathy, younger age, lower ejection fraction, longer QRS interval, or undergoing generator replacement.24,25 Data suggest that antiarrhythmic medications can affect the DFT and chronic use of amiodarone tends to increase the DFT.26,27 Further effects of antiarrhythmic medication effects on DFT are shown in Table 2.28 The question of DFT testing in patients undergoing generator replacement is complex. In a review by Phan et al., the authors concluded that DFT testing should be performed for any generator change involving a hazard alert lead, but that routine DFT testing in this situation requires further study.4 Testing allows for the potential detection of ‘silent’ lead malfunction, but the incidence of this is likely to be low. Current guidelines have a class IIa recommendation for DFT testing when there is a generator change.7
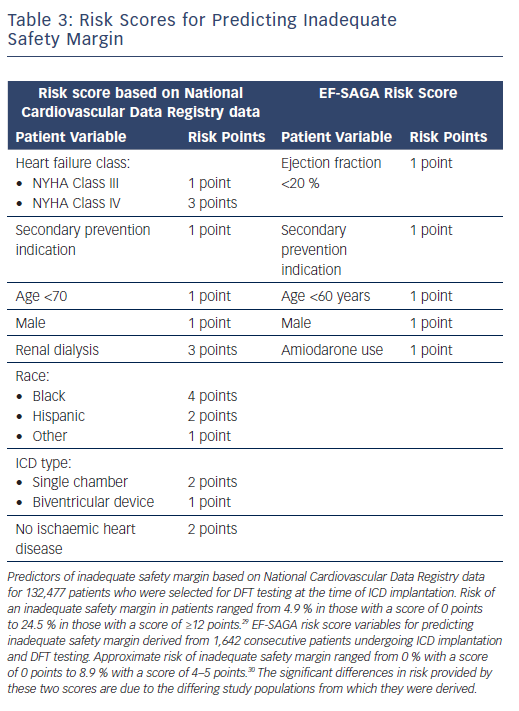
Risk scores have been developed to help clinicians estimate the likelihood of an elevated DFT or inadequate safety margin for new implantation. An analysis of the National Cardiovascular Data Registry (NCDR) of 132,477 patients who had DFT testing at the time of ICD implantation from 2010 to 2012 yielded a risk score that was able to identify patients at greater risk of an inadequate defibrillation safety margin, using a combination of eight patient-specific variables.29
The EF-SAGA risk score was developed in a retrospective analysis of 1,642 consecutive patients who underwent ICD implantation with DFT testing at the time of implant (Table 3).30 The advantages of EF-SAGA are its simplicity and ease of use. Additionally, current guidelines assign a class IIa recommendation for DFT testing in patients undergoing right-sided transvenous ICD implantation or ICD pulse generator changes, though high-quality data are lacking.7
Another important element of DFT testing is the ability to appropriately detect ventricular arrhythmias. With improvements in transvenous device sensing capabilities and algorithms including the use of band-pass filters and automatic gain control, the need to detect ventricular arrhythmias has diminished.1 Current guidelines suggest that an R wave amplitude of ≥5 mV can reliably predict a device’s ability to sense VF.7 Recent data suggest, however, that an even smaller R wave amplitude in sinus rhythm of ≥3 mV is sufficient to reliably sense VF.31 Nonetheless, DFT testing should be considered in patients with abnormal pacing, sensing, or impedance values at the time of implant according to current guidelines.7
Current guidelines also provide class III recommendations for DFT testing. Absolute contraindications to DFT testing include presence of intracardiac thrombus, AF without anticoagulation, severe aortic stenosis, acute coronary syndrome, or haemodynamic instability requiring inotropic support. Relative contraindications include severe un-revascularised coronary artery disease, recent coronary artery stent placement, recent stroke or transient ischaemic attack, and haemodynamic instability that does not require inotropic support.1,7
It should be noted that multiple observational studies have failed to show the clinical benefit of DFT testing.32–4 There are many hypotheses for this. First, the benefit of the ICD is small and accrues over time, which may make the benefit of DFT testing statistically difficult to demonstrate. Second, DFT testing occurs under controlled conditions under sedation and with medical optimisation, which is very different than what happens clinically where ventricular arrhythmias may be triggered by decompensated heart failure, electrolyte imbalance or MI. Additionally, the majority of arrhythmias are ventricular tachycardia (VT) rather than VF. For these reasons, DFT tested under laboratory conditions may be very different from what is encountered after device implantation.
Trial Data on Defibrillation Threshold Testing in Transvenous Devices
We now have randomised data which confirms the lack of clinical benefit of routine DFT testing. The yield of DFT testing has declined over the years as devices and techniques have improved and this has raised doubts over the necessity of routine DFT testing and whether it affects patient outcomes.
Two large clinical trials, the NO Regular Defibrillation Testing In Cardioverter Defibrillator Implantation (NORDIC ICD) trial and the Shockless IMPLant Evaluation (SIMPLE) trials, both published in 2015, have addressed this question.35,36 The NORDIC ICD trial was a randomised, multi-centre, non-inferiority study of 1,077 patients undergoing ICD implantation in Europe.35 Subjects were randomised to DFT testing with system revision if necessary versus no DFT testing. They were followed for 1 year with a primary endpoint of first shock efficacy. All subjects had their devices programmed to deliver 40 J regardless of DFT testing results. There was no difference in the primary endpoint of first shock efficacy for all true VT/VF episodes between the two groups. The group that underwent DFT testing had significantly more intraoperative hypotension. The trial excluded patients receiving right-sided implants or subcutaneous ICDs.
The SIMPLE trial was another randomised international, multi-centre, non-inferiority study of 2,500 patients that compared DFT testing with corresponding system modification versus no DFT testing at the time of implant.36 All subjects had their devices programmed to deliver a first shock energy of 31 J regardless of DFT testing results, and participants were followed up for an average of 1 year. The two groups had similar results related to the primary outcome of a composite of failed appropriate shock or arrhythmic death. There was no difference in overall safety outcomes between the two groups, but there were significantly more patients who received non-elective intubation in the DFT testing arm. Similar to the NORDIC trial, subcutaneous devices and right-sided implants were excluded.
These trial data demonstrated the safety of standard device programming without DFT testing at the time of transvenous device implantation, which helped lead to the current guideline recommendations.
However, data are still lacking regarding DFT testing after the initial implant. Whether to test DFT when there is a generator change or if there is a change in antiarrhythmic therapy, for example, are not addressed in the guidelines, but limited data suggest that such routine testing has a low yield.4,7,19,37 Of course, guideline recommendations are not a substitute for clinical judgement, so a patient presenting with multiple failed ICD shocks certainly warrants DFT testing and appropriate system modification (Figure 2).
Defibrillation Threshold Testing in Subcutaneous ICDs
The S-ICD has become an important treatment option for prevention of sudden cardiac death. As an entirely extravascular system, its benefits include lower risk of systemic infections, less thrombogenicity and lower levels of lead malfunction.38 Currently, the maximum output of the device is 80 J and implant testing typically is performed at 65 J, with a successful defibrillation indicating a safety margin of 15 J. Current guidelines give a class I recommendation for DFT testing during implantation of S-ICDs, and this recommendation is primarily based on a paucity of data to support the safety of foregoing DFT testing.7 Although this is the guideline recommendation, a recent analysis of NCDR data demonstrated that DFT testing at the time of S-ICD implantation is performed in 71 % of cases and is mainly performed according to preference at each setting.39
Some observational data and propensity comparisons of S-ICD outcomes versus those of transvenous ICDs have emerged in recent years. In the Evaluation oF FactORs ImpacTing CLinical Outcome and Cost EffectiveneSS of the S-ICD (EFFORTLESS S-ICD) registry, 861 patients underwent DFT testing at the time of implant, representing 93.8 % of the study population and only 0.5 % had an inadequate safety margin.40 DFT testing prompted lead repositioning in an additional 1.7 % of patients, an overall yield similar to that for transvenous devices. In a small observational study of 178 patients by Peddareddy et al., there was no significant difference in first shock efficacy among patients who had DFT testing at the time of S-ICD implant compared with those who did not.41 Randomised trials are needed to confirm this observational data and the safety of DFT testing at the time of S-ICD implant.
An important distinction with the S-ICD compared with transvenous systems is that the S-ICD has a fixed lower-sensing floor of 0.08 mV and a low high-pass filter of 3 Hz, which can present issues regarding the appropriate detection and treatment of ventricular arrhythmias. In a French study of 137 consecutive patients undergoing S-ICD implantation, 4 % had failure of the device to recognise and treat VF due to noise oversensing at the time of implant, which was resolved by changing the sensing vector.42
An emerging risk factor for inadequate safety margins in S-ICD implantation is obesity or being overweight. An analysis of the S-ICD Investigational Device Exemption study found higher BMI to be associated with a higher rate of first shock failure during device implantation.43 This also correlated with higher lead impedance measurements, presumably due to increased adipose tissue. A case report of high DFT testing and lead impedance with S-ICD lead position in the fat layer demonstrated an improved safety margin and lower impedance by repositioning the lead to just above the sternum.44 In a computer model, the optimal lead position involved placing the coil and generator directly against the fascia where there is no intervening fat.45 However, whether this optimal positioning can routinely achieve acceptable safety margins requires further research, particularly in light of a report demonstrating the inability to obtain an acceptable DFT in an obese patient despite repositioning.46 The recently developed ‘PRAETORIAN Score’ uses the posteroanterior and lateral chest X-ray to evaluate three elements of S-ICD positioning: sub-coil fat, generator position and sub-generator fat. The total score then assigns either low, intermediate, or high risk for an inadequate safety margin on DFT testing.47 These data showed a 99.6 % negative predictive value for a low-risk score in the validation cohort. The prospective evaluation of this scoring system is the subject of the PRospective, rAndomizEd Comparison of subcuTaneOus and tRansvenous ImplANtable Cardioverter Defibrillator Therapy (PRAETORIAN-DFT) trial which is currently enrolling participants.
The decision to perform DFT testing must be balanced against the fact that patients selected for an S-ICD are generally less likely to receive therapies from their devices. According to a propensity-score matched analysis of the EFFORTLESS and SIMPLE trial data, S-ICD patients received appropriate shocks 9.9 % of the time versus 13.8 % in matched transvenous ICD patients over a 3-year follow-up time.48 This is similar to another propensity-matched study, which found that appropriate ICD therapy was significantly higher in transvenous device patients.49 Theories as to why this discrepancy exists include that the S-ICD has a longer charging time, thus allowing more ventricular arrhythmias to self-terminate, and patients in these cohorts were selected for a S-ICD if they were thought to not have benefited from antitachycardia pacing therapy. These differences between the use of S-ICD and transvenous ICD therapy will affect the risk/benefit ratio when considering whether or not to perform DFT testing.
Our Approach
In general, our decision to perform DFT testing at the time of implant is guided by the risk scores discussed above, as well as current guideline recommendations. In general, we perform DFT testing in patients receiving ICDs for secondary prevention or right-sided implants where we feel the benefit of DFT testing outweighs the risk. Otherwise, for standard left-sided device implantations with acceptable sensing (R wave amplitude ≥5 mV), pacing and impedance values we do not perform DFT testing. We also do not routinely perform DFT testing at the time of generator change unless there is a hazard alert lead or other pacing, sensing, or impedance values which might indicate lead failure. For S-ICD implantation, we perform DFT testing in all patients in the absence of contraindications.
When DFT testing is indicated, we coordinate with the anaesthesia service for monitored anaesthesia care or general anaesthesia. For testing, we set the maximum programmable device sensitivity and induce VF with either rapid ventricular pacing at rates up to 50 Hz or with low-energy shock delivery during the vulnerable period of ventricular repolarisation, according to the operator’s discretion. The method of DFT determination is operator dependent (Figure 1), as no particular method has been shown to be better than any other. After a first successful defibrillation, a second induction is performed to confirm the DFT or safety margin.
Conclusion
Routine DFT testing is no longer recommended at the time of implantation of left-sided transvenous ICDs, a recommendation supported by findings from randomised trials. It is not unreasonable to consider DFT testing in selected patients, depending on the risk of an inadequate safety margin based on established risk scores such as the EF-SAGA or the NCDR-derived scores. While observational data suggest that DFT testing at the time of S-ICD implant may not be necessary in all patients, there is no randomised data to support this practice. Until such data become available, DFT testing should be performed at the time of S-ICD implant according to guideline recommendations.
Clinical Perspective
- Defibrillation threshold (DFT) testing is not routinely recommended during implantation of left-sided transvenous ICD, which is supported by randomised trial data.
- The presence of patient-specific risk factors may prompt DFT testing according to operator discretion at the time of implantation in certain clinical situations.
- Current guidelines recommend DFT testing should be done at the time of subcutaneous ICD (S-ICD) implantation in the absence of contraindications. Limited data are available for DFT testing in S-ICD implantation and more robust studies are needed to demonstrate the safety of omitting the test.