With the advent of successful surgical repairs and modern diagnostic techniques, an increasing number of patients with congenital heart disease survive to adulthood. Despite these improvements, the surgical corrective atrial incisions performed during childhood lead to subsequent myocardial scarring that have the inherent risk of harbouring substrates for macro-reentrant atrial tachycardias (MRATs). Apart from surgical conduction barriers such as patches, conduits and surgical scars, there are also natural barriers including valve annuli and venous orifices that potentially may become critical substrates for tachyarrhythmias.1 The 20-year risk of developing atrial arrhythmias among patients with adult congenital heart disease (ACHD) was 7 % in a patient aged 20 years and 38 % in a patient aged 50 years in a population-based study.2
MRATs are the most common type of supraventricular tachycardia (SVT) in patients with ACHD, occurring in 75 % of ACHD-associated SVT cases, most commonly in patients with Ebstein’s anomaly, Tetralogy of Fallot, single-ventricle Fontan procedures, transposition of the great arteries (TGA) or atrial septal defects (ASDs).3,4 Over 60 % of these MRATs involve the cavo-tricuspid isthmus (CTI) and are referred to as CTI-dependent atrial flutters, while the other MRATs are defined as non-CTI-dependent atrial flutters.3 In addition, some forms of ACHD are associated with tachyarrhythmias occurring prior to any surgical intervention, such as Ebstein’s anomaly with up to 44 % prevalence of atrioventricular (AV) or atriofascicular accessory pathways and congenitally corrected TGA with AV accessory connections in 2–5 % of patients.5
Apart from these tachyarrhythmias, patients with ACHD also have an increased risk of pump failure, stroke and sudden cardiac death (SCD).6 Although the incidence of SCD is low (0.09 % per year) in the CHD population, it is higher compared with age-matched controls7 and related to arrhythmias in 14 % of all deaths after initial repair.8,9 Atrial tachycardias (ATs) and impaired ventricular function are thus important and consistent risk factors for SCD in patients with ACHD, of which those with corrected Tetralogy of Fallot, post-atrial switch operation (Mustard or Senning), left heart obstructive lesions and univentricular hearts have the highest risk of SCD (2–9 % per decade).8–10 In the German National Register for Congenital Heart Defects for adult patients, the mortality rate during a median follow-up of 3.7 years was 9.2 % among 2596 patients, with heart failure (27.6 %) and SCD (23.0 %) as the leading causes of death.11 Deceased patients had a more complex CHD and extracardiac comorbidities.
The treatment of CHD imposes certain safety considerations when choosing antiarrhythmic drugs while various cardiac anatomical barriers and required operator expertise should be taken into account when referring patients for catheter ablation. The complex cardiac anatomy and haemodynamic changes require special precautions related to the increased risk of pro-arrhythmia when using antiarrhythmic drugs and the need for specialised expertise and sophisticated mapping systems when complex catheter ablation procedures are performed. Pre-procedural cardiac imaging with a variety of methodologies can assist with venous access to the heart, determining details of native cardiovascular anatomy and the nature of corrective surgery and evaluation of ventricular function. Reviewing all tachycardias from prior ECGs is also important before the procedure. The success rates after catheter ablation in patients with ACHD is somewhat lower compared with the general population, with acute and long-term success rates of 80 % and 68 %, respectively, for accessory pathway ablations, and 66–76 % and 50–53 %, respectively, for MRATs.12–15
As recommended for patients with AF, antithrombotic therapy is indicated in patients with ACHD who have AT or MRAT.16–18 Expert recommendations for physicians managing patients with ACHD is mandatory due to the small number of patients and the potentially life-threatening arrhythmias.
This review summarises current evidence-based developments in the field, focusing on new advances and general recommendations for the management of patients with ACHD, including published recommendations on management of SVT19 that has adopted a new user-friendly system of ranking a recommendation using heart symbols in three different colours.
Diagnosis and Differential Diagnosis of Supraventricular Tachycardia
Traditionally SVT (defined as an atrial and/or ventricular rate >100 beats per minute [bpm] at rest involving tissue from the His bundle or above) includes AV reentry tachycardia (AVRT) due to accessory connections, AV nodal reentry tachycardia (AVNRT) and various forms of ATs including focal atrial tachycardias and MRATs.19,20 Most SVTs are regular and may manifest as narrow-QRS tachycardias (QRS duration <120 ms) or wide-QRS tachycardias (QRS duration ≥120 ms). Regular and paroxysmal palpitations with a sudden onset and termination are most likely related to AVRT or AVNRT. Termination by vagal manoeuvres further suggests a reentrant tachycardia involving AV nodal tissue. Preexcitation on the surface ECG in a patient with regular paroxysmal palpitations strongly suggests AVRT, whereas irregular palpitations suggest AF or non-sustained atrial tachycardia.
Narrow QRS Tachycardias
An ECG recorded during tachycardia is of key importance for an adequate diagnosis of SVT.21 Focal atrial tachycardia is an organised atrial rhythm usually ranging between 100 and 250 (rarely, up to a maximum of 300) bpm and the diagnosis is clear when the ventricular rate is lower than the atrial rate. An automatic AT is characterised by gradual acceleration of the atrial rate at tachycardia onset (warmup phenomenon) and deceleration (cool down) before termination. Irregular R-R intervals during a tachycardia are consistent with AF if discernible P waves are absent, whereas although atrial flutter with varying degrees of conduction may also be irregular, it is often marked by a recurring pattern of ‘grouped beating’. During AT the conduction to the ventricles can be fast (1:1) or slow (3:1 or 4:1), but a 2:1 conducting atrial flutter should be strongly suspected for patients with ACHD with palpitations and seemingly inappropriately high heart rate, especially when there is a strong history of sinus node dysfunction. Although a discrete P wave with an intervening isoelectric interval suggests a focal AT, an MRAT cannot be excluded in a patient with significant ACHD.22
Vagal manoeuvres or adenosine injection may aid in clinical diagnosis (see Table 1). AV dissociation during narrow-QRS tachycardia excludes AVRT, as both atria and ventricles are parts of the circuit.
Wide-QRS Tachycardias
Wide-QRS tachycardias are most commonly (80 %) ventricular tachycardias (VTs), but can be SVT with bundle-branch block (BBB) aberration (15 %) or conduction over an accessory pathway (5 %).23 Diverse BBB morphology during wide-QRS tachycardia compared with sinus rhythm strongly favours VT. Functional BBB is more frequently right sided because of its longer refractoriness. An accessory pathway may participate in the reentry circuit (antidromic AVRT) or act as a bystander during SVT (AT, atrial flutter, AF and AVNRT). Differential diagnosis should always be considered in the context of the underlying disease, with conditions such as Tetralogy of Fallot favouring VT.
A 12-lead ECG during wide-QRS tachycardia showing ventriculo-atrial (VA) dissociation (atrial activity slower and independent of ventricular activity), is a major ECG criterion for VT. A 1:1 VA conduction is found in up to 50 % of patients with VT and gives no diagnostic clue. The QRS morphology during wide-QRS tachycardia may be useful for diagnosis in the absence of VA dissociation, although conventional criteria may not apply as VT morphology has not been systematically evaluated in this population.1,23 The differentiation between VT and antidromic AVRT is difficult unless the surface ECG during sinus rhythm shows the same preexcitation pattern. SVTs conducted with aberrancy or antidromic AVRT respond to vagal manoeuvres and adenosine, as described for narrow-QRS tachycardia (see Table 1).
Clinical Presentation
The clinical presentation of SVT depends on heart rate, blood pressure during the tachycardia, type of cardiac malformation and corrective surgery, and the individual patient symptom threshold. The symptoms may vary from an asymptomatic patient to a state mimicking panic disorders with breathlessness or to a serious symptom such as syncope.24 A clinical history including description of symptoms and precipitating factors with number of episodes, duration, frequency, mode of onset and an evaluation of cardiac function is mandatory. Patients with ACHD are more likely to present with breathlessness or chest discomfort/pain, particularly at fast heart rates of >150 bpm, as compared with patients without structural heart disease. Prolonged asymptomatic SVT episodes may lead to a tachycardiomyopathy if left untreated for weeks to months, with a fast ventricular rate.25
Common Types of Supraventricular Tachycardia in Adult Congenital Heart Disease
Macro-reentrant Atrial Tachycardias
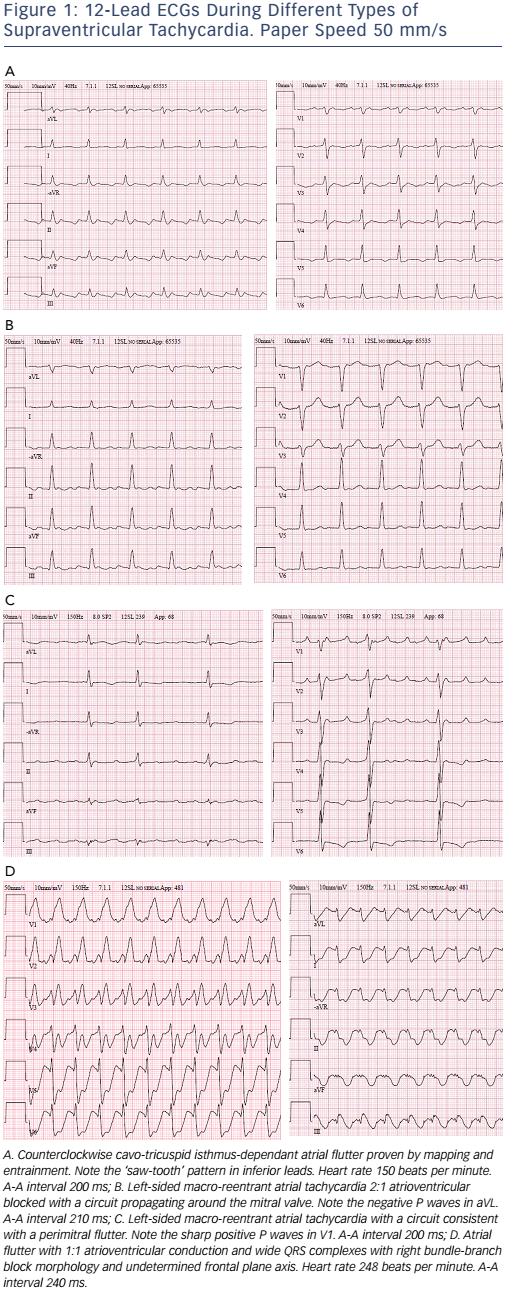
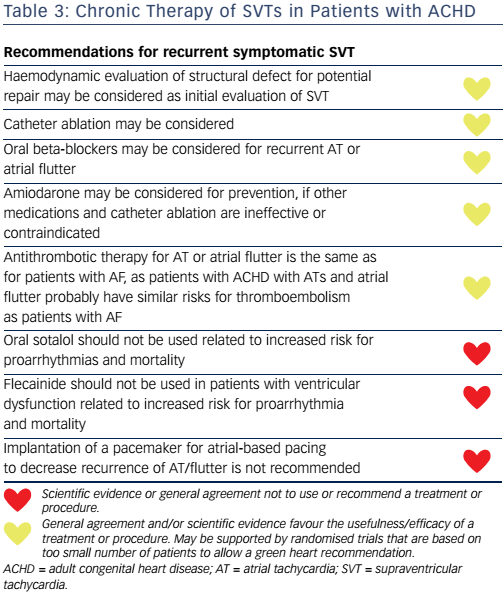
CTI-dependent atrial flutter is an MRAT in the right atrium that propagates in counter-clockwise direction with regular negative atrial deflections in inferior leads as a ‘saw-tooth pattern’, at rates of 240–350 bpm (see Figure 1).26 It propagates in the clockwise direction less often, resulting in positive atrial deflections in inferior leads and bimodal negative waves in V1. CTI-dependent right atrial flutter is a common tachycardia mechanism in patients with postsurgical ACHD. Although a typical, saw-tooth ECG pattern is seen in inferior leads, other MRATs should be considered.27–29 The relatively narrow passage, the CTI, is the preferred ablation target.
Non-CTI-dependent atrial flutters can occur with cycle lengths as long as 400 ms, and with more than one ECG pattern, suggesting several tachycardia mechanisms.30 Right-sided MRATs include a ‘lower-loop’ circuit, considered to be a variant of CTI-dependent atrial flutter at a lower level than usual with its ECG pattern, described after the Fontan operation31 and Mustard/Senning repair,32 and ‘upper-loop’ circuits around the superior vena cava and upper portions of the crista terminalis or, more commonly, atriotomy circuits at the lateral right atrial surgical scars or a septal patch.29 In patients with complex ACHD undergoing the Mustard, Senning or Fontan procedures, complex suture lines and large areas of scar tissue result in substrates for MRAT, but the CTI is, in many cases, the critical isthmus for the reentry circuit, but with difficult access for ablation.32,33
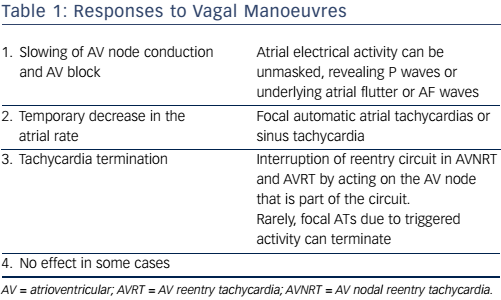
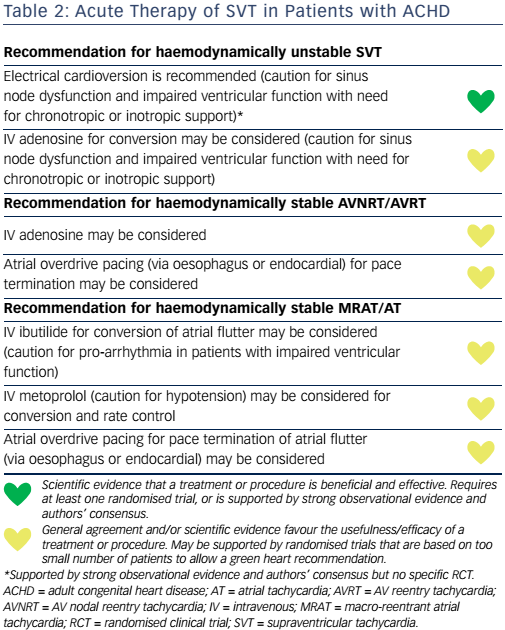
Left-sided MRATs (see Figure 1) are generally related to gaps along surgical lines and anatomical barriers, such as the mitral valve or the pulmonary veins29 and can be interrupted by localised ablation at those sites, but with a lower success rate and a higher recurrence rate than CTI ablation.34–39 Catheter ablation requires complete lines of block, preferably at the narrowest isthmus, which can be challenging, particularly at the mitral isthmus.
Prevention of macro-reentry at cardiac surgery may be achieved by connecting large atriotomies to electrical obstacles whenever possible, to avoid creating new reentry circuits. The superior septal approach to the left atrium should therefore be avoided.
Focal Atrial Tachycardias
Focal ATs usually have frequent interruptions and re-initiations as opposed to MRATs, which are stable. The ablation strategy for focal AT, irrespective of the mechanism, targets the site of earliest activation from which it spreads centrifugally to the rest of the atria, whereas the strategy for MRATs follow a large circuit around a central obstacle, usually a scar or an anatomical barrier.40 Atrial rates above or below 240 bpm and the presence or absence of an isoelectric baseline between atrial deflections are traditionally useful diagnostic features, but do not discriminate focal from macro-reentry mechanisms as activation in slow conduction tracts may not be recorded in the ECG.26,41 Thus, focal and reentrant ATs can thus only be distinguished by activation and entrainment mapping during an electrophysiological study.22 Although a negative P wave in lead aVL usually implies a left atrial origin while a negative in V1 supports a source in the lateral right atrium, the value of the P-wave morphology during tachycardia for assessing the origin of an AT is also limited in patients with ACHD (see Figure 1).
Atrioventricular Reentrant Tachycardias
Wolff–Parkinson–White (WPW) syndrome refers to the presence of preexcited ECG during sinus rhythm in association with recurrent tachyarrhythmias. AF with fast ventricular response is a potentially life-threatening arrhythmia in patients with WPW syndrome due to degeneration in ventricular fibrillation. Predictors of SCD in patients with WPW syndrome include:
- Short R-R interval (<250 ms) during spontaneous or induced AF;
- History of symptomatic tachycardia;
- Multiple accessory pathways;
- Ebstein’s anomaly.42,43
Acute Therapy of Supraventricular Tachycardia in Adult Congenital Heart Disease
Acute treatment of SVT in patients with ACHD should be preceded by an immediate clinical evaluation for efficient and safe treatment, including:
- Evaluation of haemodynamic status and the cardiovascular disease;
- Effect of previous acute treatments;
- Current medical therapy;
- Duration of the ongoing tachycardia.
Acute treatment alternatives for SVT, shown in Table 2, have been reported in European guidelines.19 A tachycardia should be documented with a 12-lead-ECG before acute management unless the patient is severely haemodynamically unstable, when at least a rhythm strip should be recorded. Direct current (DC) synchronised cardioversion is the most effective method and first choice to terminate any haemodynamically unstable narrow or wide QRScomplex tachycardia.44–47 Vagal manoeuvres (i.e. Valsalva) should be used in the first attempt to terminate a tachycardia,48–50 during which an ECG should be recorded as the response may aid the diagnosis even if the arrhythmia does not terminate (see Table 1).
Intravenous (IV) administration of adenosine (5–15 mg in a bolus) is the drug of first choice in haemodynamically stable patients if vagal manoeuvres fail.51,52 The advantages of adenosine include a rapid onset, short half-life, and avoidance of hypotension, which makes it the drug of choice except for in patients with severe asthma or angina pectoris.51 Adenosine may induce AF (1–15 %), which may be of concern for those with ventricular preexcitation, although the AF is usually transient.
A short-acting beta-blocker (esmolol) is the second choice for acute tachycardia termination in haemodynamically stable patients (see Table 2).51,53,54 Longer-acting agents (e.g. IV metoprolol) may be preferred in patients with frequent premature beats, which may trigger early recurrence of paroxysmal supraventricular tachycardia. IV ibutilide given for conversion of atrial flutter is usually highly effective and may be considered, although caution is advised for proarrhythmia in patients with impaired ventricular function. IV calcium-channel blockers are not recommended as they may cause hypotension and/or bradycardia. A wide QRS-complex tachycardia of unknown mechanism should be treated as VT until otherwise proven. Adenosine may aid the diagnosis or interrupt an adenosinesensitive VT, but caution is advised in case of AF with ventricular preexcitation.55 DC cardioversion is recommended for termination of any irregular wide QRS-complex tachycardia.
Chronic Pharmacological Therapy of Supraventricular Tachycardia in Adult Congenital Heart Disease
There are no randomised clinical trials evaluating the efficacy and safety of antiarrhythmic drugs (AADs) in patients with ACHD. Medication with beta-blocking agents may protect from rapid 1:1 AV conduction and tachycardia-mediated hypotension, but their preventive efficacy in SVT is uncertain (see Table 3). Non-use of betablockers was an independent predictor of appropriate implantable cardioverter defibrillator shocks in a multicentre cohort study of patients with TGA and intra-atrial baffle repair (hazard ratio 16.7; P=0.030).56 All AADs have an increased risk for proarrhythmia and many also aggravate sinus node dysfunction as well as heart failure and require in-hospital observation. Sinus node dysfunction may require pacemaker implantation prior to initiation of AADs.
In the retrospective studies comparing AAD for prevention of SVT in patients with ACHD, only 45 % of the patients were free from SVT after 2.5 years of follow-up.57 Class III AADs sotalol and amiodarone were the most effective, but adverse effects were common (22 %). Class Ic drugs (encainide and flecainide) should not be used in patients with ACHD due to their proarrhythmic effects (see Table 3).58 The recent Cochrane Database System Review of randomised trials regarding the safety of AAD compared with controls in adult patients with AF showed that all AADs except amiodarone, dronedarone and propafenone were associated with an increased risk of proarrhythmia.59 Quinidine, disopyramide and sotalol were additionally associated with increased all-cause mortality rates.59 There is no reason to expect these drugs to be safer in a population with structural heart disease, such as ACHD, and therefore they cannot be recommended otherwise than as a last-choice therapy. Amiodarone is less often associated with proarrhythmia, but has severe side-effects (thyroid and pulmonary toxicity) that limit long-term use in these patients.59 Atrial-based pacing does not seem to prevent subsequent atrial arrhythmias according to multivariate analysis.60
Catheter and Surgical Ablation
Catheter ablation of SVT is more complicated in patients with ACHD due not only to the nature of the MRAT per se, but also to the challenge with limited venous access to the heart, fibrotic atrial tissue, multiple atrial reentrant circuits, and atrial baffles separating the coronary sinus and CTI to the systemic venous atrium. Patients should preferably be referred to experienced centres with respect to complex MRATs and with access to advanced mapping systems as special expertise and knowledge of complex tachyarrhythmias and scar-related ablation procedures is required for a successful outcome (see Table 3).
Catheter ablation is further challenged by the difficult access to the pulmonary venous atrium in patients who have undergone Fontan or atrial switch procedures. Trans-baffle access was reported to be successful in 96 % of 74 attempted cases without indications of higher incidence of adverse events.61 Trans-conduit puncture for patients who have undergone extra-cardiac Fontan procedures has more recently been reported without complication related to the puncture procedure.62 Remote magnetic navigation may be especially useful for complex venous anomalies such as intra-atrial baffle or interrupted inferior venous access for access to the pulmonary venous atrium.63 Post-ablation monitoring and prospective registries and studies are, however, warranted.39,61
The acute success rates of catheter ablation of SVT in patients with ACHD ranges from 65 to 100 %, with a higher recurrence rate (20–60 %) within 2 years than seen in other cohorts of routine SVT ablation.13,64–66 Lower success rates (65–82 %) have been reported for AT or MRAT ablations as compared with those observed in the absence of ACHD,13,64,67 although better outcomes have been achieved with the advent of advanced mapping and ablation techniques.63 Although ablation of CTI-dependent atrial flutter has a high acute success rate of 96 %, depending on the type of anomaly, the recurrence rate after 45±15 months’ follow-up is as high as 18 %.68 The main factors related to higher success rates of catheter ablation in these patients are:
- Severity of ACHD (patients with single ventricle or dextro-TGA [d-TGA] with poorer outcomes);
- Age (worse outcomes with older age at repair); and
- Anatomical mapping systems and irrigated tip ablation catheters (higher success rates if used).
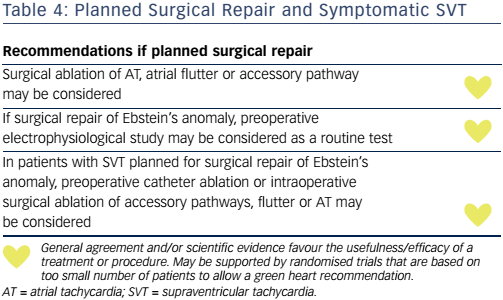
Arrhythmia surgery can be integrated into a corrective surgical procedure with high efficacy and with no obvious signs of increased surgical morbidity (see Table 4).69 A 5 % mortality rate was, however, reported with Fontan conversion when surgery was performed purely for refractory supraventricular arrhythmia.70
Specific Disease Conditions
Atrial Septal Defect
Most MRATs occurring in patients without prior closure of the ASD are CTI dependent and susceptible to catheter ablation.3 The ASD closure unlikely eliminates the atrial flutter and catheter ablation of the CTI is therefore the recommended approach.71 If the ASD mandates a closure, MRAT ablation prior to closure should be considered. Significant ASDs in adults can even be closed later in life and result in improved morbidity and survival rates,72 although new or recurrent ATs are frequent.73 It is therefore preferable to perform both catheter ablation of the AT and closure of the ASD in patients with significant ASD and tachyarrhythmia. Both CTI-dependent atrial flutters and MRATs can occur and coexist in the same patient with repaired ASD, and catheter ablation has favourable outcomes.3,65 Both transcatheter closure and surgical closure of secundum ASDs have been reported to yield favourable long-term outcomes without significant differences with regard to atrial arrhythmias (9.3 %), survival or thromboembolism.3,74,75 Patients with unoperated significant ASD and arrhythmias should therefore undergo both ablation of the AT and closure of the ASD. The anatomical features of the ASD determine the most suitable choice between catheter and surgical treatment approach. No randomised trials have compared catheter-based versus surgical closure of ASD combined with arrhythmia intervention.
Ebstein’s Anomaly
Accessory pathways are frequent (15–30 %), more often right sided and multiple in patients with ACHD than in other patients,76 and other SVTs that can occur include AF, atrial flutter and focal AT. The haemodynamic consequences of SVTs depend on the degree of malformation, varying from mild variants without any symptoms to severe haemodynamic compromise and cyanosis in cases of tricuspid regurgitation and large ASD. Preexcited AF or rapidly conducting MRATs may result in SCD. Catheter ablation of accessory pathways is challenging and associated with lower success rates (80 %) and higher recurrences (40 %) than in other patients, also depending on accessory pathway location.77 When surgical corrections are warranted and SVTs are present ablation is still recommended prior to surgery.77 Surgical ablation of accessory pathways is successful in 92–100 %.5,78 Preoperative electrophysiological evaluation has a high diagnostic and therapeutic yield and is recommended as a routine preoperative test for this population.79 Patients who underwent corrective surgery for Ebstein’s anomaly with preoperative electrophysiological study and intraoperative arrhythmia ablation had a lower risk of SCD than patients without arrhythmia intervention, in a small series.80 Catheter ablation procedures should be performed by experienced physicians related to the complex anatomy and the more complex arrhythmias.81,82
Transposition of the Great Arteries with Post-atrial Switch Operation (Mustard or Senning)
There is a high prevalence of both MRAT (14–24 %) and sinus node dysfunction in patients with TGA with post-atrial switch operation (Mustard or Senning), related to the extensive atrial surgery. Maintenance of sinus rhythm is desirable as SVTs have been associated with increased risk of sudden death, and recurrences are common and often associated with haemodynamic compromise. The number of suitable AADs is limited related to ventricular dysfunction and risk of proarrhythmia, as well as sinus node dysfunction. Catheter ablation of AT and AVNRT in patients post-Mustard or Senning operation for d-TGA has a high primary success rate, but high recurrence rates (30 %) of MRAT, although with favourable longterm results after a second ablation.66 Catheter ablation procedures are complex and should be performed in experienced centres using sophisticated mapping systems. The CTI is a critical area that is bisected by the atrial baffle, thus the portion of the isthmus connected to the tricuspid valve must often be reached either by a retrograde transaortic or an antegrade trans-baffle approach to achieve isthmus block.
Tetralogy of Fallot
In patients with Tetralogy of Fallot, MRAT is common (20 %) as is sustained VT (11 %), with an 8 % risk of SCD.4,83,84 The MRATs are drug-refractory and/or severely symptomatic in most patients and approximately half of the sustained and symptomatic cases are CTI dependent.84 The occurrence of SVTs was recently reported to be associated with higher mortality rates (15.6 % versus 8.6 %; p=0.001).85 In the majority of patients, a right BBB is present during sinus rhythm, which may cause differential diagnostic difficulties requiring electrophysiology testing.86 MRAT can be a sign of worsening ventricular function and a haemodynamic evaluation of the repair is therefore warranted. Complete or partial control of the arrhythmia can be achieved by surgery or catheter-based haemodynamic revision of the repair in cases who are clinically and haemodynamically ill.87 Catheter ablation of AT is associated with a high procedural success rate during long-term follow-up in the vast majority of patients.88
Fontan Repairs
Despite more modern surgical approaches, the incidence of atrial arrhythmia remains high. The most common AT in patients who have undergone a Fontan procedure is an MRAT (66 %), and CTI-dependent atrial flutter or AF can develop in up to 42 % of patients.89 ATs can rapidly cause haemodynamic deterioration resulting in heart failure. Catheter ablation is often difficult due to multiple circuits and should be attempted only at experienced centres. When compared with other ACHD anomalies, both Fontan and Mustard repairs have been associated with less successful ablation results related not only to the low success rate of catheter ablation (54 % versus 83% for other CHDs), but also to a high recurrence rate (50 % versus 32 %) after an initial successful ablation procedure.64,67 Lower recurrence rates were achieved with modified right atrial maze procedures as compared with CTI ablation,90 but selection of suitable patients is important.91
Summary
The prolonged survival of patients with ACHD has imposed further challenges for the adult electrophysiologists who are expected to manage even more complex MRATs. A rapid evolution of electroanatomical mapping systems with developments in computer technology and sophisticated ablation equipment have not only improved the mapping, but enhanced the understanding of specific arrhythmia mechanisms, appreciation of scar tissue and transmurality of ablation lesions, which should improve our efficiency and result in more controllable and safer procedures while treating these patients. Despite these novel evolving techniques, further development of prophylactic intraoperative ablation techniques at the time of congenital heart surgery is urgently needed to prevent subsequent MRATs.
Clinical Perspective
- ACHD is a structural heart disease that imposes restrictions for safety reasons when choosing antiarrhythmic drugs related to the increased risk of proarrhythmias.
- ACHD is further characterised by a large variety of cardiac anomalies and anatomical barriers resulting in complex macro-reentrant atrial tachycardias that require advanced knowledge and expertise for effective and safe catheter ablation procedures.